CROI 2025 Prevention Round-up

The science offered at the Conference on Retroviruses and Opportunistic Infections (CROI) 2025 is a showcase of the great promise and importance of prevention research. With broadside attacks and a sweeping funding withdrawal by the US government affecting science and global health, these advances in the field make clear how much is at stake. Innovation in long-acting PrEP and new evidence to support on-demand PrEP among women could transform global health, if the field can unite behind the evidence and refuse to be defeated.
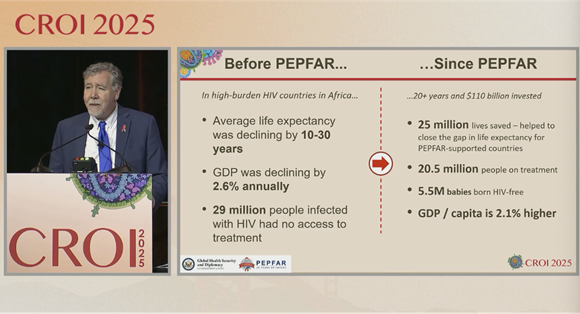
New data presented during the opening plenary underscores these dire realities: nearly one in five children under one with HIV who experienced a treatment interruption in 2024 died, based on a review of over half a million children in US-funded PEPFAR programs. These findings emphasize the critical need to maintain uninterrupted care for young children with HIV, who are especially vulnerable to rapid disease progression, and starkly highlight the catastrophic risks associated with halting funding for treatment programs.
Read below for more important scientific highlights. And read this new piece outlining the promise of next-generation HIV prevention, the challenges posed by the new US administration, and new resources to secure a future for PrEP research, development, and access.
The Power of LEN for PrEP
A once-yearly injection of lenacapavir (LEN) for PrEP took a step forward. Renu Singh of Gilead Sciences presented data from an ongoing, open-label study of the pharmacokinetics (how a drug is absorbed, distributed and eliminated in the body at a given dose), safety and tolerability of two LEN formulations testing an intramuscular formulation. Singh reported that drug concentrations were as good as or better than the 6-monthly subcutaneous dose that showed 100% efficacy in 2024.
The doses were safe and well tolerated. A Phase III study is expected to launch later this year, with possible regulatory submissions in 2027. Gilead’s application for twice-yearly LEN for PrEP is currently under FDA priority review with a decision expected by June 19. (ViiV Healthcare is similarly advancing their four-month injectable cabotegravir (CAB) towards possible regulatory submission, while the two-month formulation is rolling out.)
More data from the PURPOSE Trials were presented showing that a cohort of 16-17 year-old females were just as well protected by 6-month injections of LEN for PrEP as adults in the larger study. Katharine Gill of the Desmond Tutu HIV Foundation, South Africa credited the PURPOSE trials and Good Participatory Practice for the landmark study, the first large-scale trial to include adolescents in the initial study design. Despite having high HIV incidence, adolescents have historically been excluded from Phase III HIV trials, resulting in prolonged delays in access to PrEP.

On Demand PrEP for Cisgender Women: 3 days or 4?
Findings from a modeling study explored how to optimize an on-demand protocol for HIV protection in the female genital tract. On-demand PrEP, sometimes called event-driven PrEP or 2-1-1, is a CDC approved protocol for men who have sex men who opt to take PrEP over a three-day period, starting 2-24 hours before the time of a specific sexual exposure, then one pill every 24 hours for the following two days. Mackenzie Cottrell of the University of North Carolina reported findings that adding a 4th day of dosing heightened protection from vaginal exposure to HIV.
Dosing of 2-1-1-1 showed 84% protection, 2-2-2-2 showed 95% protection. Cottrell said, “While limited data suggest 2-2-2-2 dosing is safe for short-term use, the 2-1-1-1 regimen may better balance safety, efficacy, and tolerability while maintaining effectiveness.” Cottrell recommended the 2-2-2, 2-1-1-1, and 2-2-1-1 regimens be considered in future clinical studies of on-demand PrEP in cisgender women.
This data builds on the analysis of a body of evidence, led by Jeanne Marrazzo, now director of NIAID, showing that oral PrEP can reliably prevent HIV infection in cisgender women even with non-perfect adherence.
MK-8527: A Monthly PrEP Pill
Merck presented an analysis of animal and Phase I data that informed the MK-8527 doses chosen to be tested in its ongoing Phase II PrEP trial. Looking at protection provided in monkeys, as well as pharmacokinetic data in humans, Merck determined that a monthly dose of at least 6 mg would provide adequate protection in more than 90% of the population intended for MK-8527 use. The Phase II trial is testing 3mg, 6mg, and 12mg doses in participants with low risk of HIV exposure. Watch this space for further information about how the Phase II trial will inform the upcoming efficacy program of MK-8527 expected to start later this year.
F/TAF Works in Women With Medium-to-High Adherence
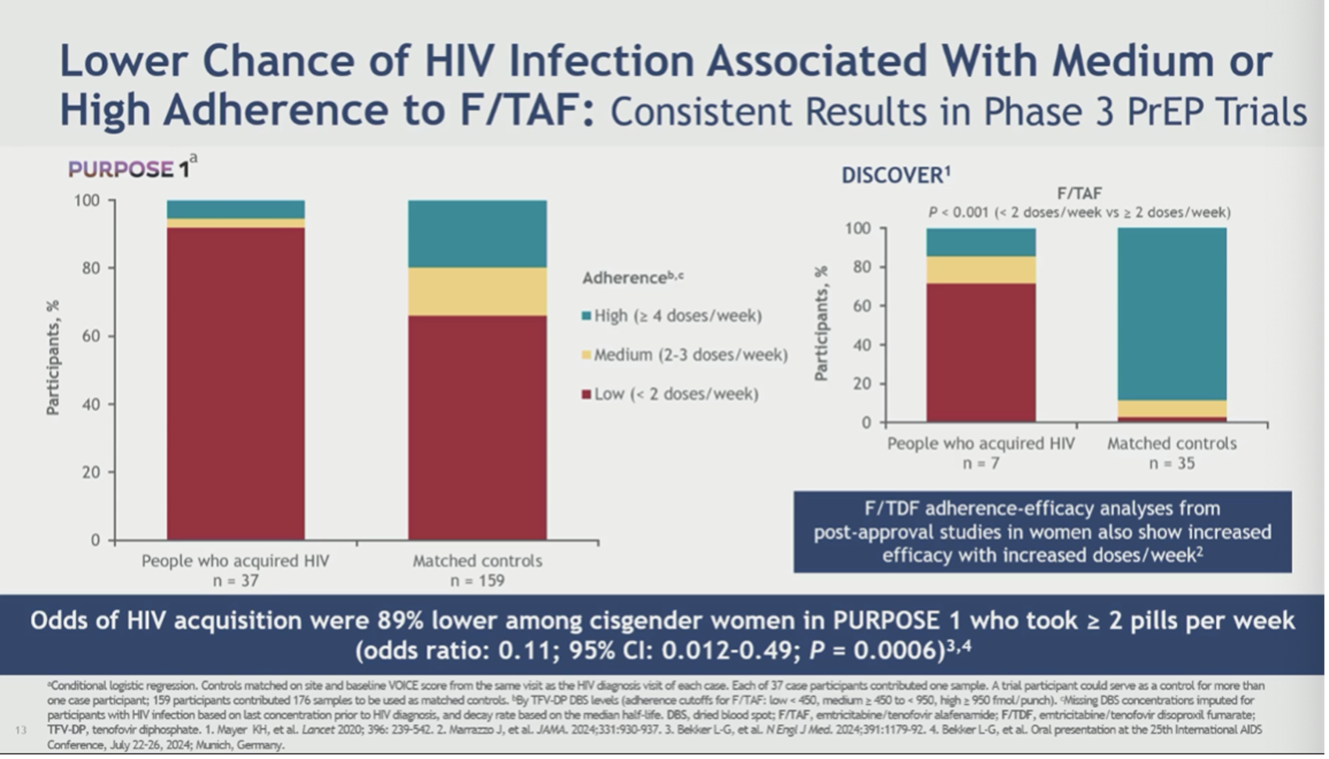
Flavia Kiweewa of Makerere University-Johns Hopkins University Research Centre in Uganda presented important new prevention evidence from the PURPOSE 1 trial that tested both injectable LEN and F/TAF among cisgender women for PrEP. This new analysis found the chance of acquiring HIV was 89% lower when adherence to F/TAF reached two pills per week or more.
Kiweewa reported that “nearly all incident HIV cases in participants receiving F/TAF in PURPOSE 1 were attributable to low oral PrEP adherence…taken together, these results suggest that HIV infections in PURPOSE 1 occurred almost always in the context of nonadherence to F/TAF, with rare emergence of HIV resistance and low risk of HIV diagnosis delay.” Kiweewa concluded that F/TAF is another prevention tool that should be considered for women who prefer a daily oral HIV prevention option.
For more CROI 2025 highlights, including on cure and STI prevention, see avac.org’s dedicated page.