Dapivirine Vaginal Ring Early Market Access Vehicle (EMAV)

In response to the voices of adolescent girls and women who need more HIV prevention choices, The Global Fund (GF) and the Children Investment Fund Foundation (CIFF) have partnered to make dapivirine vaginal rings available in GF-supported countries through an early market access vehicle.
See here for EMAV request form in English. For guidance on how to complete the form, see the EMAV launch webinar (slides / recording).
If you require resources in another language, please contact [email protected] for support.
Objective: Facilitate immediate access to one-month ring to accelerate availability for users, expand PrEP choice, and catalyze impact while less expensive rings come to market with an approximate 60% drop in price/month.
Mechanism Summary:
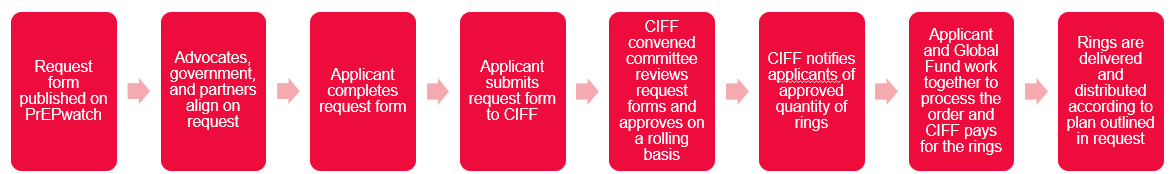
Eligible Applicants: Country entities with HIV product procurement and supply chain responsibilities who may or may not be GF principal recipients or sub recipients in countries which received an HIV allocation for GC7. Endorsement by National Ministry of Health or other government agency responsible for the HIV response is required. Product registration is not required if import waiver can be obtained.
Available Volumes: 2,400 (min.) and 24,000 (max.) one-month rings, per country, for use across 2025 and 2026. Available rings will be allocated on a first-come first-served basis to requests which meet the criteria below. CIFF will fund up to 150k rings in total. Out of this total, there are currently 103,200 rings remaining to be allocated. This page will continue to be updated as volumes are allocated.
Deadline: Requests are accepted on a rolling basis after launch October 30.
Monitoring and Evaluation: Quarterly reporting to CIFF and leveraging reporting of GF and PEPFAR, when applicable.