The first ARV developed for PrEP was a daily pill comprised of a two drug combination of tenofovir disoproxil and emtricitabine (TDF/FTC). It was first approved in 2012, and is now approved for all populations at risk of HIV. In some countries, tenofovir disoproxil is combined with lamivudine (TDF/3TC), a drug with a similar chemical structure to emtricitabine that shares the same efficacy against HIV and can be less expensive. TDF/FTC and TDF/3TC are often collectively referred to as TDF/xTC.
The safety and efficacy of TDF/FTC and TDF/3TC is well-established. Policy and program decisions are guided by both data from the original randomized clinical trials, as well as newer studies. Demonstration projects, implementation initiatives and evidence from national programs have provided information about challenges and opportunities with oral PrEP delivery, and can help optimize services and identify gaps.
Advocacy
Prevention options that mitigate or eliminate barriers to access are essential. Key populations, including men who have sex with men (MSM), transgender women (TGW), female sex workers (FSW), and adolescent girls and young women (AGYW) need effective options that work and fit into their lives. Each HIV prevention option has unique characteristics, and individuals may prefer a particular PrEP method for any number of reasons.
Ensuring informed choice is key. Oral PrEP is another strategy to help reduce HIV risk. For some people it will be the right one; for others, injectable cabotegravir for PrEP, the dapivirine vaginal ring (the PrEP ring) or another non ARV-based approach will be right. Research and development have created new options; now advocacy is needed to make them viable choices for people who need and want them.
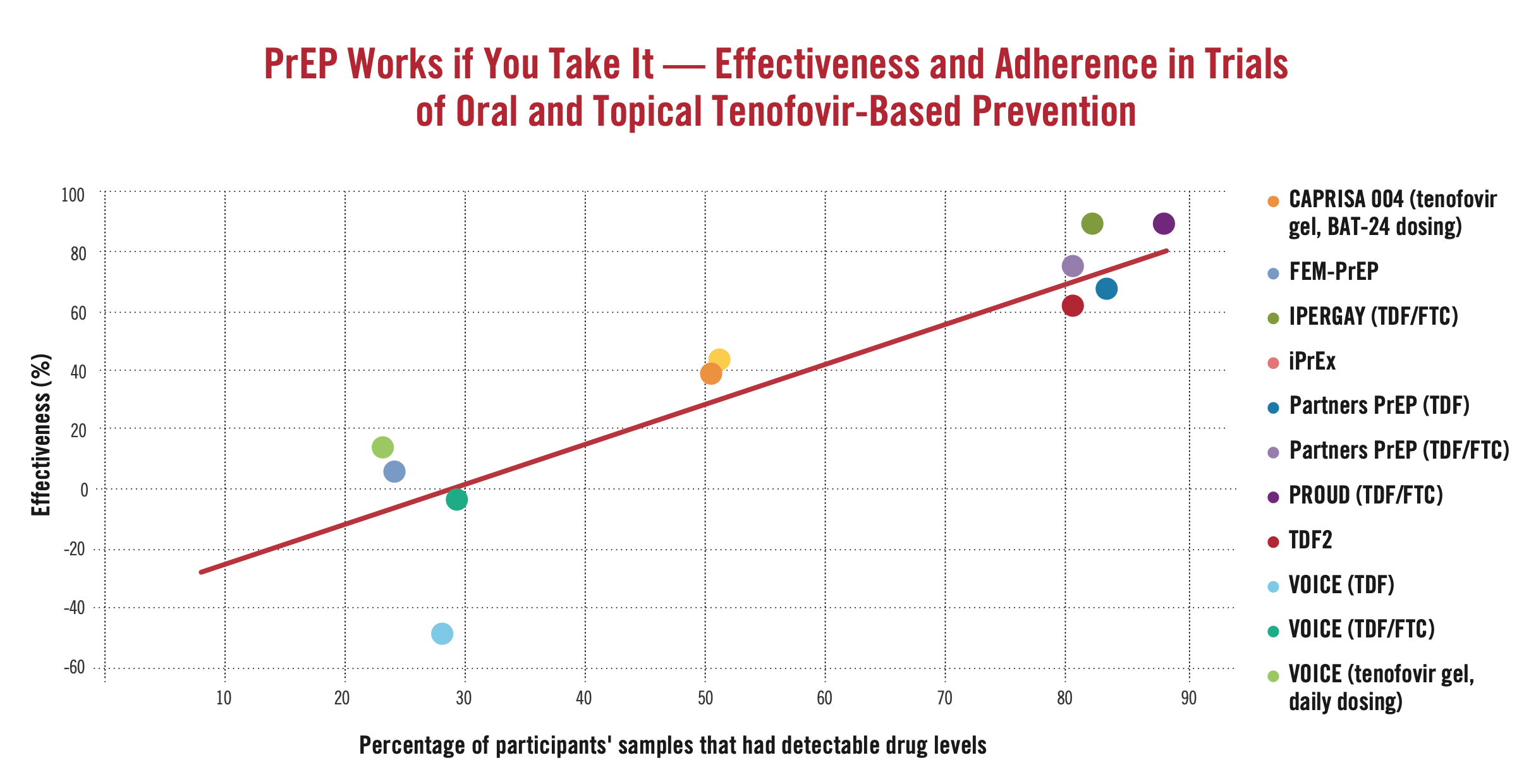
Clinical Trials Evidence
The 2012 US FDA decision and WHO guidance on PrEP demonstration projects were based on data from randomized controlled trials of TDF/FTC and TDF (a generic option). Additional randomized trials since then have added to this evidence base.
- Table of Findings by Trial – where they took place, who enrolled, what they found
- Adherence Matters – summary of randomized controlled trial data on the relationship between adherence and efficacy
- PrEP Fact Sheet – includes a narrative description of randomized controlled trials to date
Ongoing Research
PrEPVacc
The PrEPVacc trial is simultaneously testing experimental HIV vaccines and oral PrEP against a placebo. Participants are randomized to receive one of two experimental vaccines, which have both been tested before but did not advance to late-stage trials on their own. PrEPVacc is evaluating if they work better in combination. Participants are also randomized to receive one of two types of daily oral PrEP: TDF/FTC or F/TAF. Participants receive the daily oral PrEP over weeks 0–26; vaccine injections are received over weeks 0–48. This Phase IIb trial is enrolling 1,668 men and women, aged 18-40, from Mozambique, South Africa, Tanzania, and Uganda.
Findings from National Introduction and Scale-Up
Visit AVAC’s Global PrEP Tracker for the latest updates to PrEP uptake by country.
For lessons from the field on what works and what innovation is underway check out the resources section from the Prevention Market Manager Project, our report Lessons from Oral PrEP Programs and their Implications for Next Generation Prevention, and the following issue briefs that highlight key findings: