Back to Injectable Lenacapavir for PrEP
Injectable Lenacapavir for PrEP
Injectable lenacapavir (LEN) is an investigational antiretroviral (ARV) drug that is being studied as a potential PrEP product. Below are details on its distinct characteristics and ongoing Phase II and III clinical trials, as well as resources to learn more.
The Basics
- Given every six months.
- Two subcutaneous injections given on the same visit in the abdomen.
- Developed by Gilead.
- Approved in multiple countries as treatment under the brand name Sunlenca.
- Currently under investigation for use as prevention in two large Phase III clinical trials, PURPOSE 1 and PURPOSE 2. Early results from PURPOSE 1 showed no HIV infections in the LEN for PrEP arm, and early results from PURPOSE 2 showed only two infections in the LEN for PrEP arm.
- In December 2024, Gilead submitted an application to the US Food and Drug Administration (FDA) for approval for use as HIV prevention. The US FDA has granted Gilead a priority review, which means an approval decision will be made by 19 June 2025.
- Gilead has also submitted LEN for PrEP to the European Medicines Agency, which covers all countries in the European Economic Area, and to the South African Health Products Regulatory Authority.
- Three smaller Phase II clinical trials- PURPOSE 3, PURPOSE 4, and PURPOSE 5- are investigating safety and efficacy in populations not included in PURPOSE 1 and 2.
- A one-year version of LEN for PrEP is also under investigation. Early results have been positive, and a Phase III clinical trial is expected to launch later in 2025.
The Trials
PURPOSE 1
- Conducted among approximately 5,000 cisgender women in South Africa and Uganda.
- Testing the efficacy of both LEN for PrEP and the daily pill emtricitabine/tenofovir alafenamide (F/TAF) in preventing HIV.
- In June 2024, the trial was unblinded after meeting its primary endpoint of superiority to oral PrEP (TDF/FTC) and background HIV incidence.
- Scheduled to run until July 2027.
- The first Phase III clinical trial to include pregnant and lactating people from the start—which could make it easier to get approval for use in this population if found to be effective and safe.
- Read a summary here and the full results from the New England Journal of Medicine here.
PURPOSE 2
- Conducted among 3,000 men who have sex with men, gay men, transgender men, transgender women, and gender non-binary people in Argentina, Brazil, Mexico, Peru, Puerto Rico, South Africa, Thailand, and the USA.
- In September 2024, the trial was unblinded after meeting its primary endpoint of superiority to oral PrEP (TDF/FTC) and background HIV incidence.
- Scheduled to run until April 2027.
- Read a summary here and the full results from the New England Journal of Medicine here.
PURPOSE 3
- Conducted among 250 cisgender women in the USA.
- Scheduled to run until January 2028.
PURPOSE 4
- Conducted among 250 people who inject drugs in the USA.
- Scheduled to run until July 2027.
PURPOSE 5
- Exact population and timeframe still to be announced, but will be conducted among people who may benefit from but are not yet taking PrEP in France and the United Kingdom.
Further Resources
- Tracking Lenacapavir Rollout — interactive graphics outlining immediate next steps on pathways to access and impact, key actors responsible for them, timelines for completion, and important progress updates
- The Gears of Lenacapavir for PrEP Rollout
- From Clinical Trial Efficacy to Public Health Impact: A plan for accelerating access to injectable lenacapavir for PrEP
- The Lens on LEN — AVAC’s primer on lenacapavir
- Summary of PURPOSE 1 trial and results
- AVAC’s press release on the PURPOSE 1 trial interim results
- Gilead’s press release on the PURPOSE 1 trial interim results
- AVAC’s press release on the PURPOSE 2 trial interim results
- Gilead’s press release on the PURPOSE 2 trial interim results
- View the AVAC infographic, An Overview of Lenacapavir for PrEP Trials
- Gilead’s statement on access planning in high-incidence, resource-limited countries for lenacapavir for HIV prevention
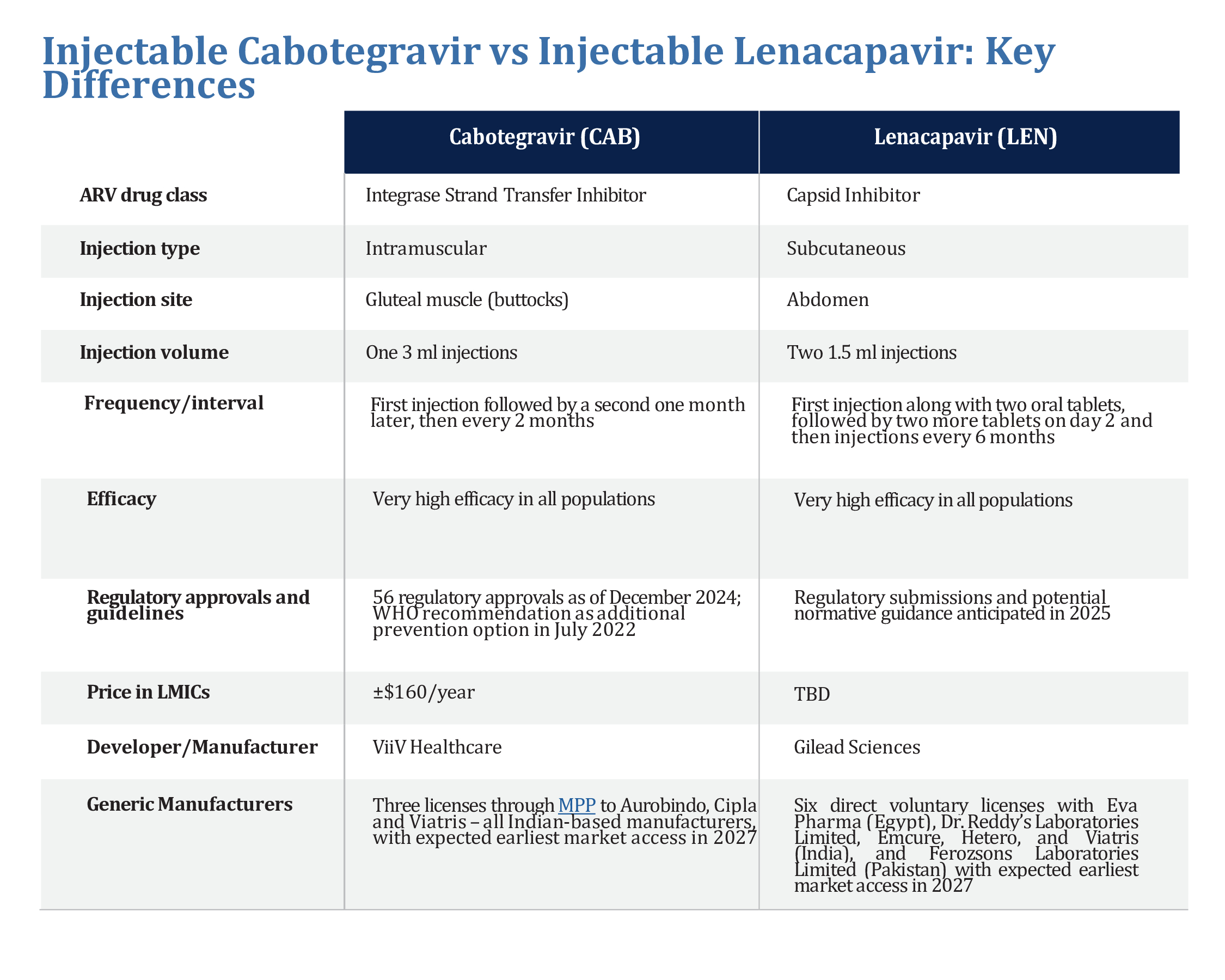
The graphic below compares lenacapavir with another injectable PrEP product, cabotegravir. Click here to download the graphic.