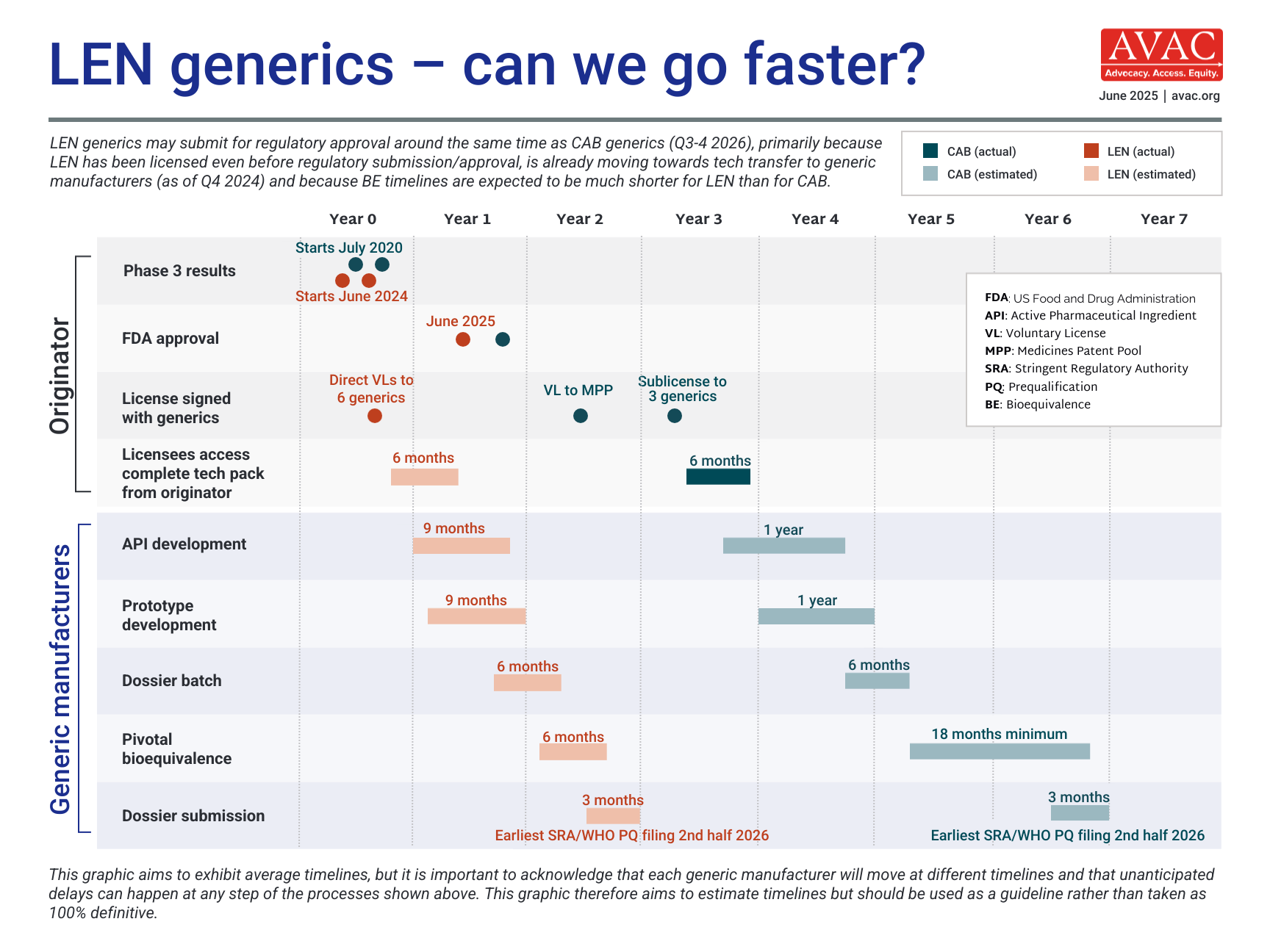
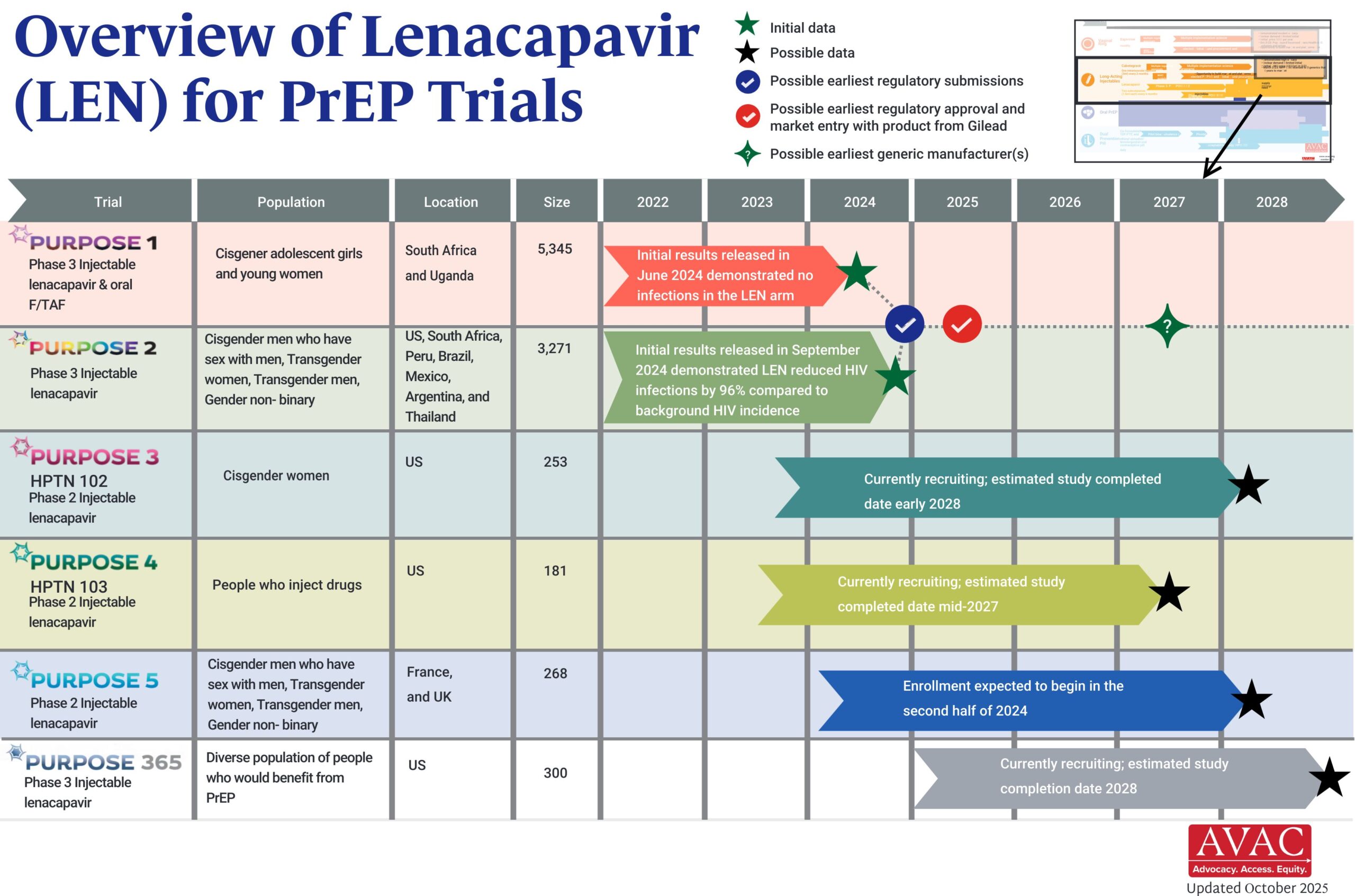
Long-Acting PrEP Status Update
Updated as of January 2026
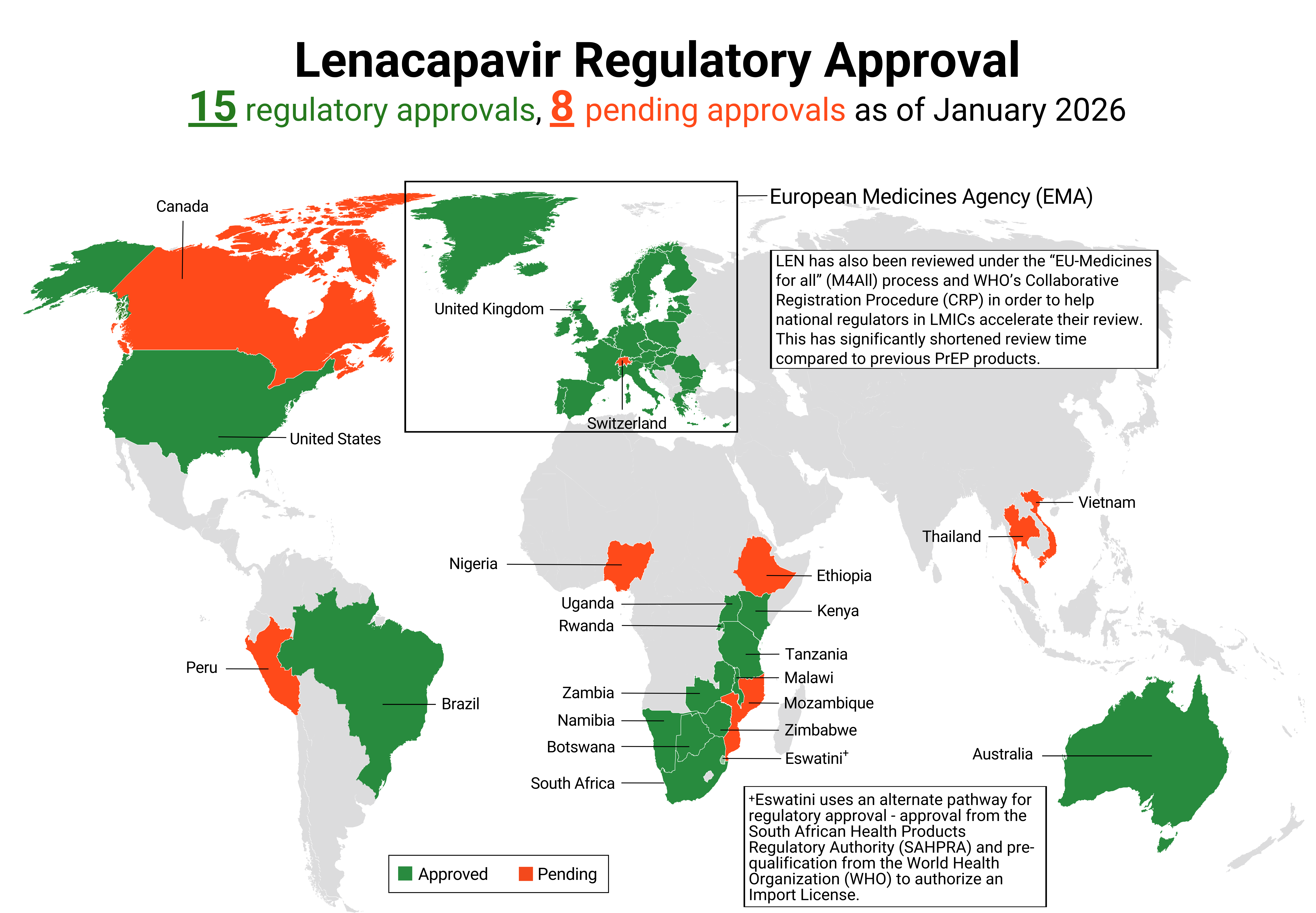
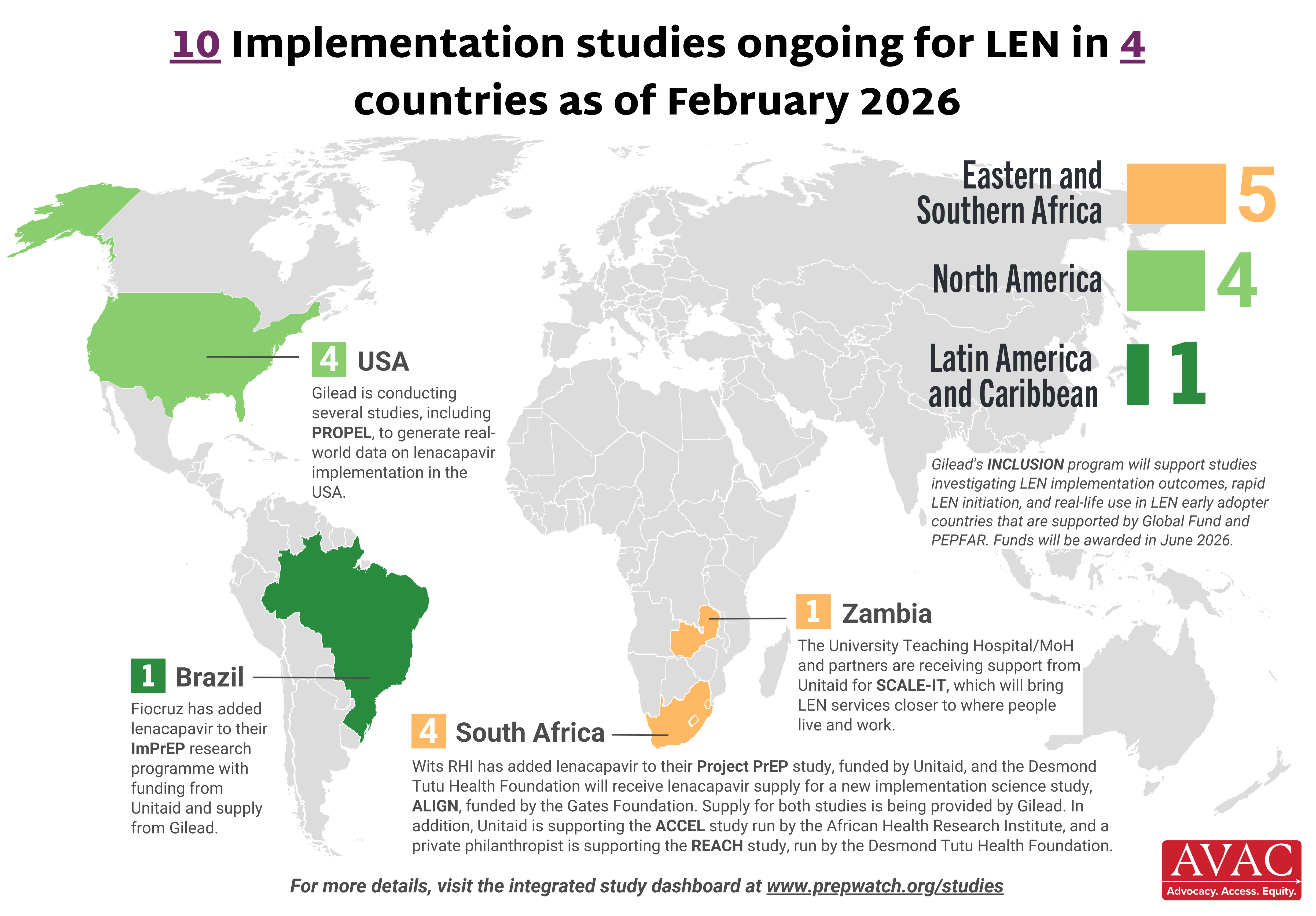
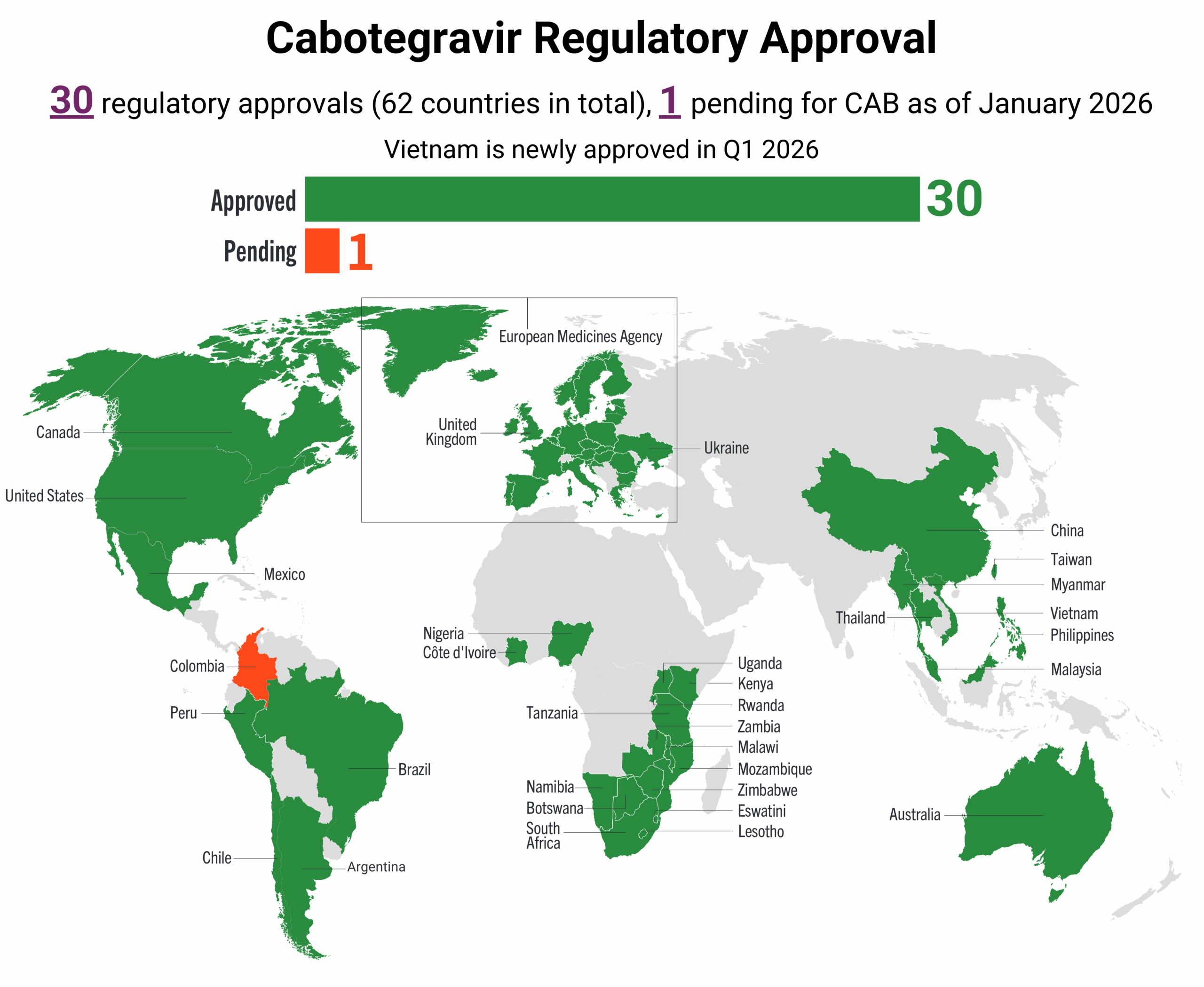
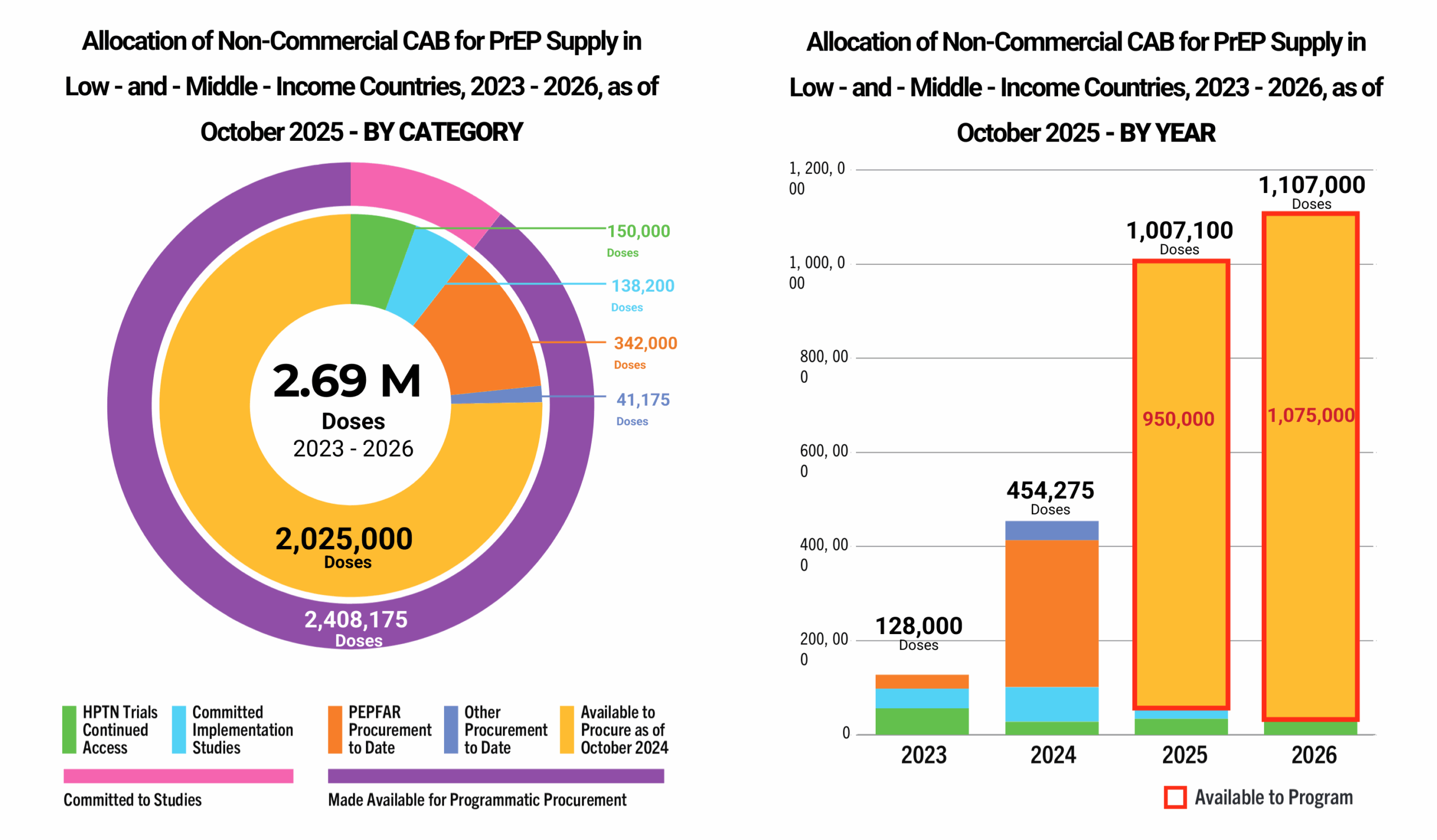
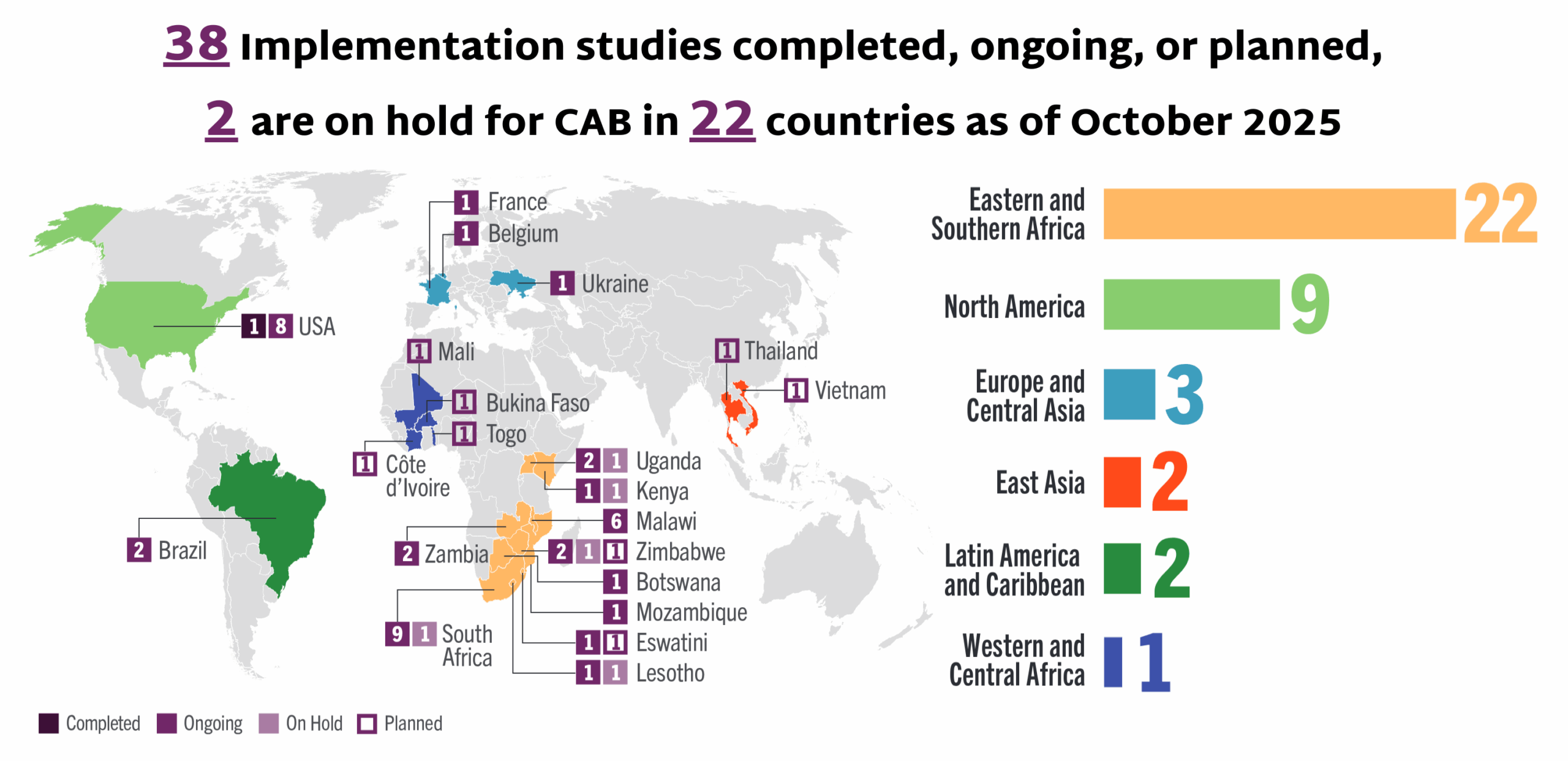
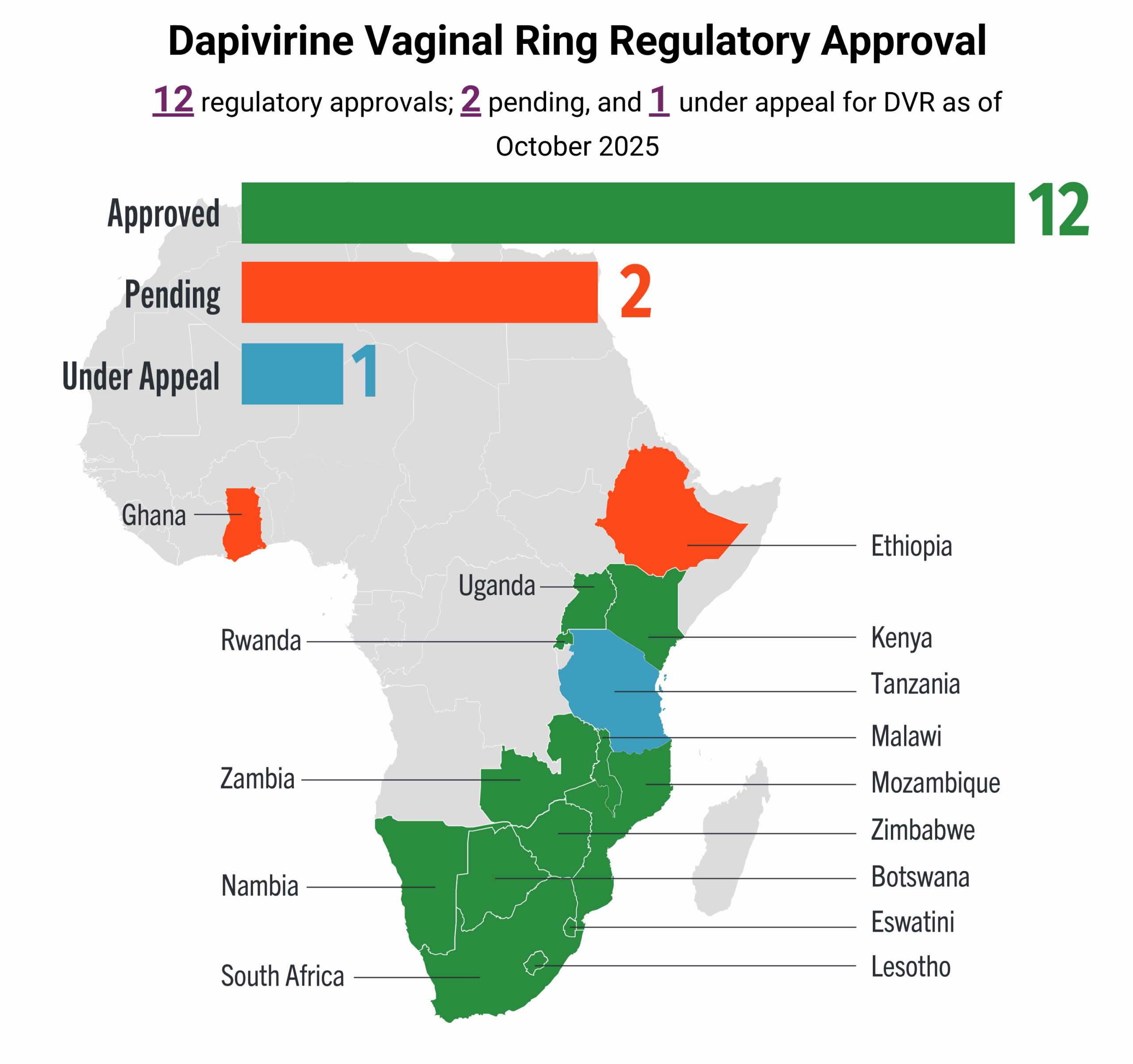
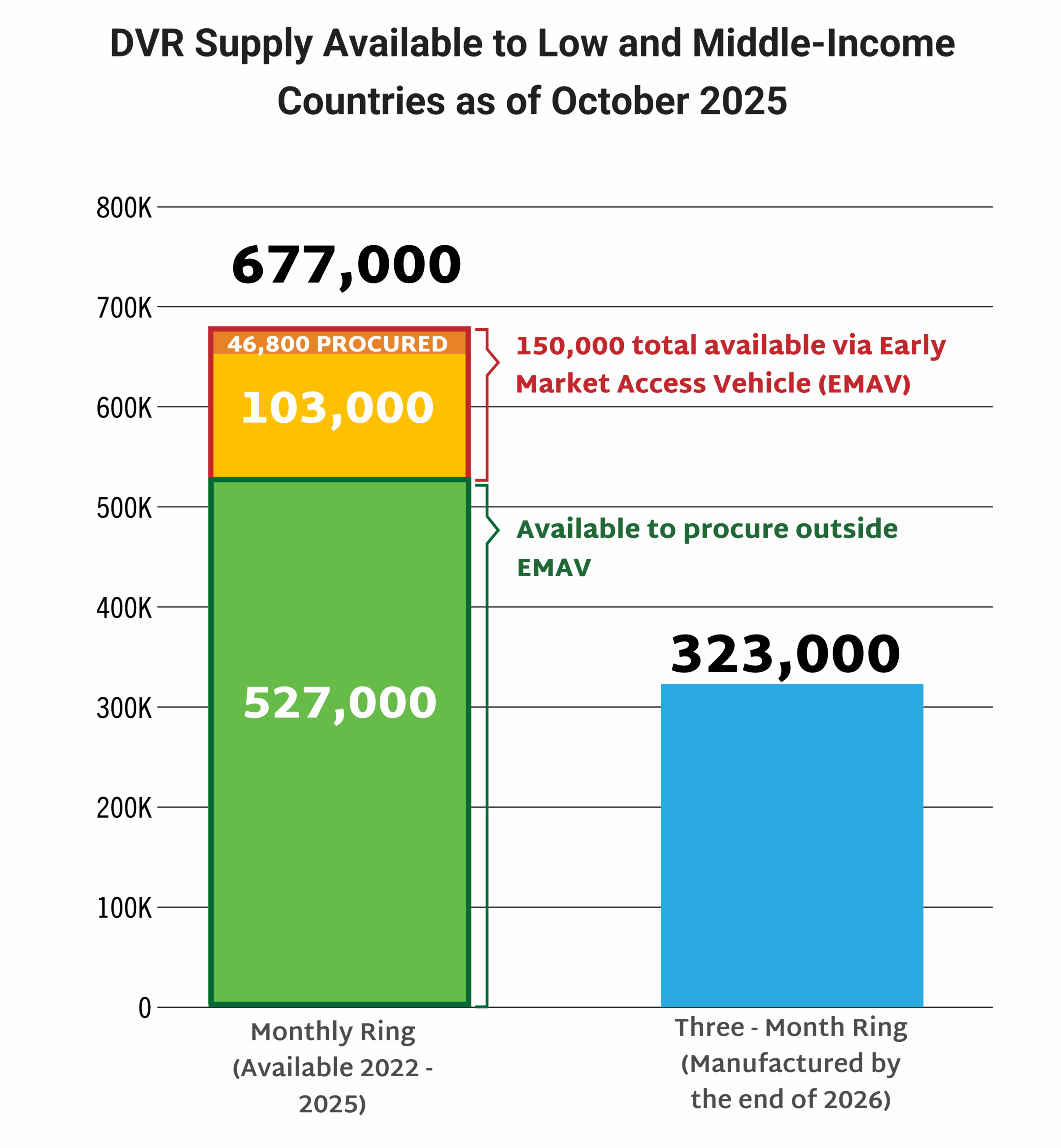
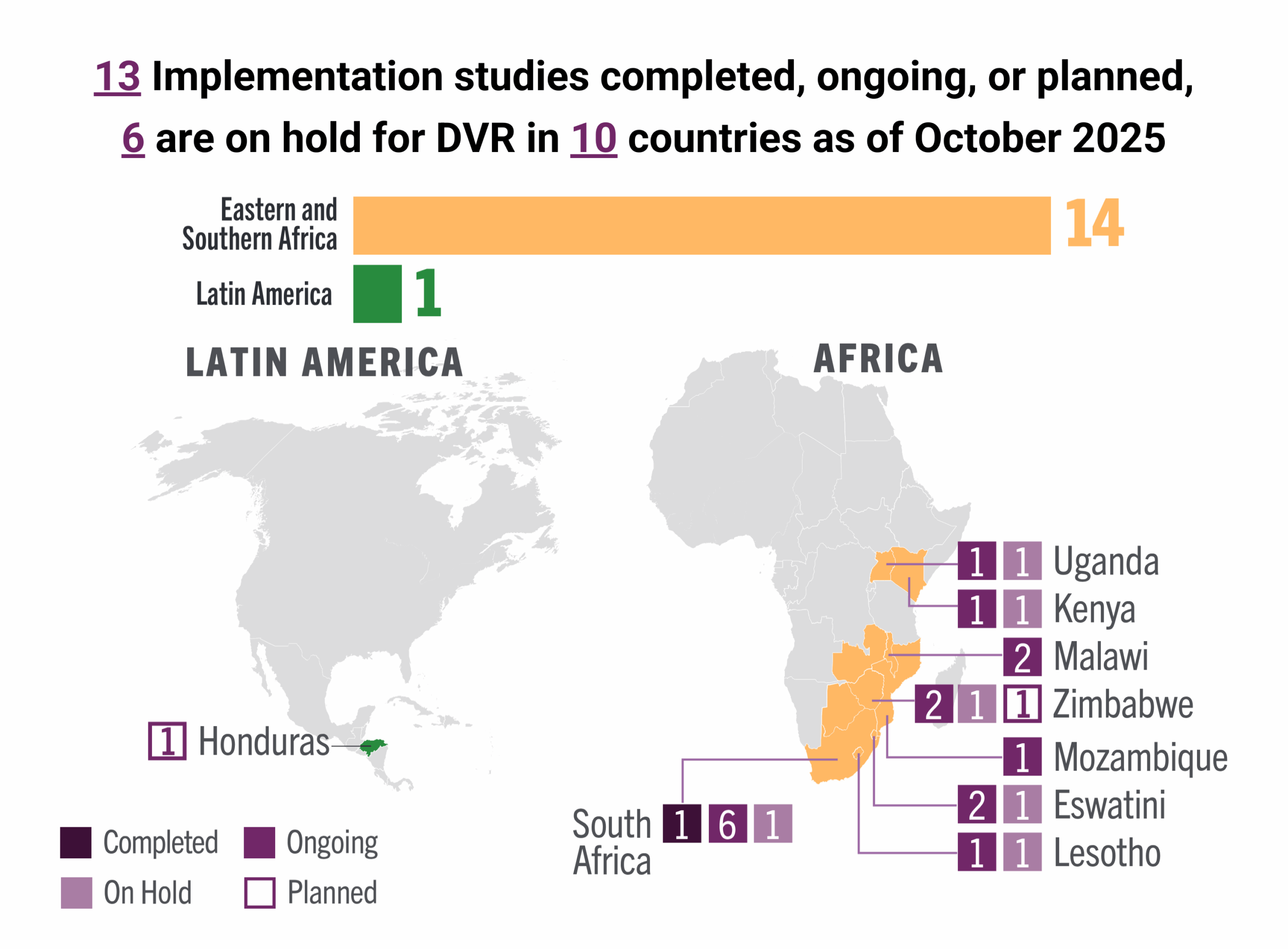
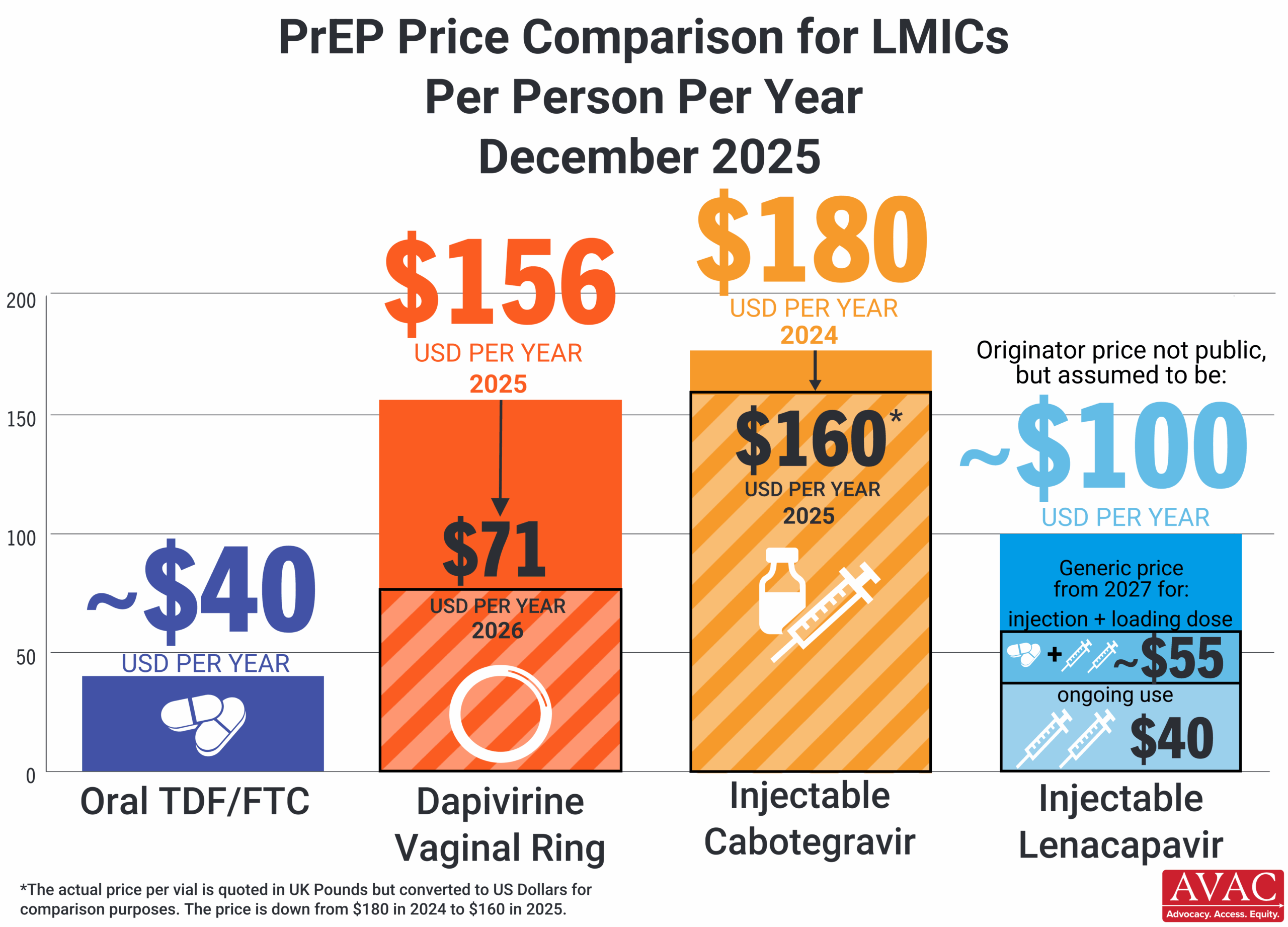
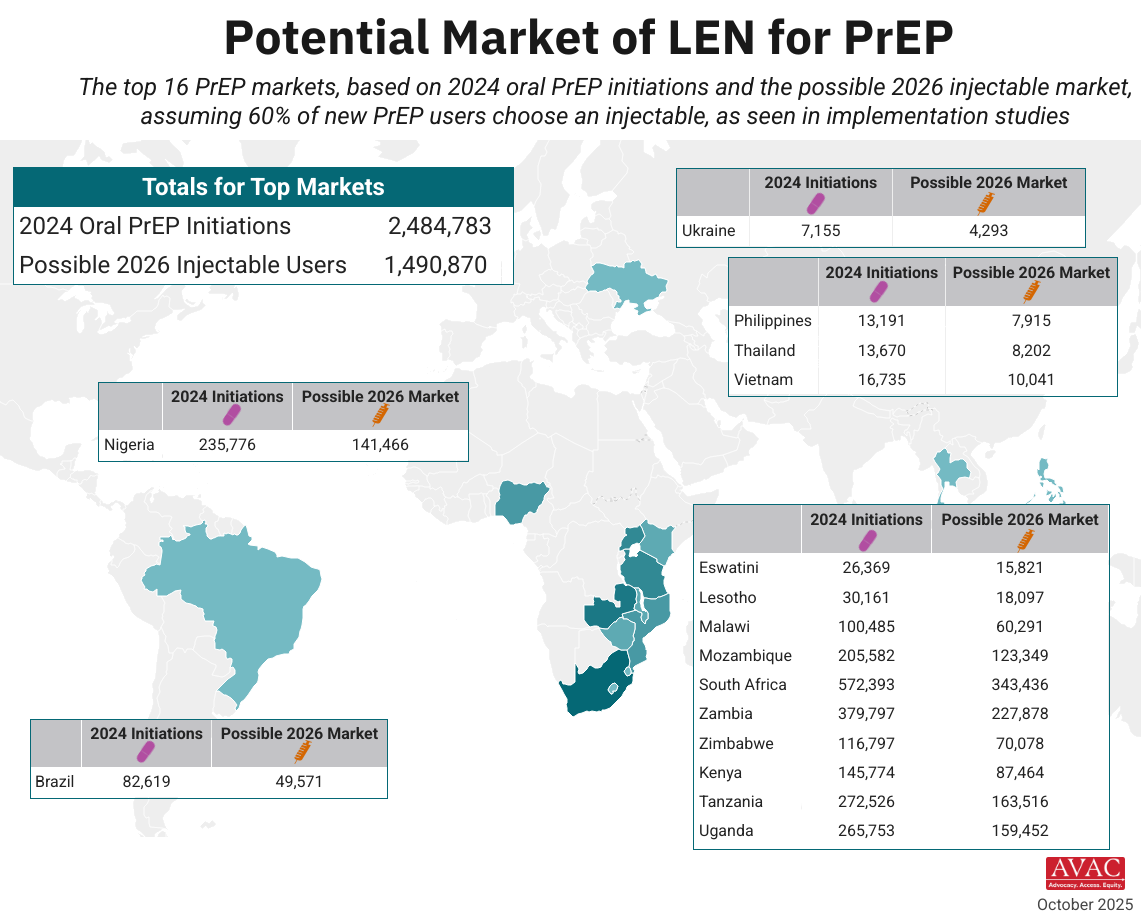
This dashboard provides a timely snapshot on the progress of accelerating access to long-acting PrEP products. The graphics featured here track regulatory approvals, volumes, ongoing and planned implementation science studies, and non-profit prices for approved longer-acting PrEP products, including injectable cabotegravir, injectable lenacapavir, and the dapivirine vaginal ring. The dashboard is updated quarterly by AVAC, Secretariat to the Coalition to Accelerate Access to Long-Acting PrEP.
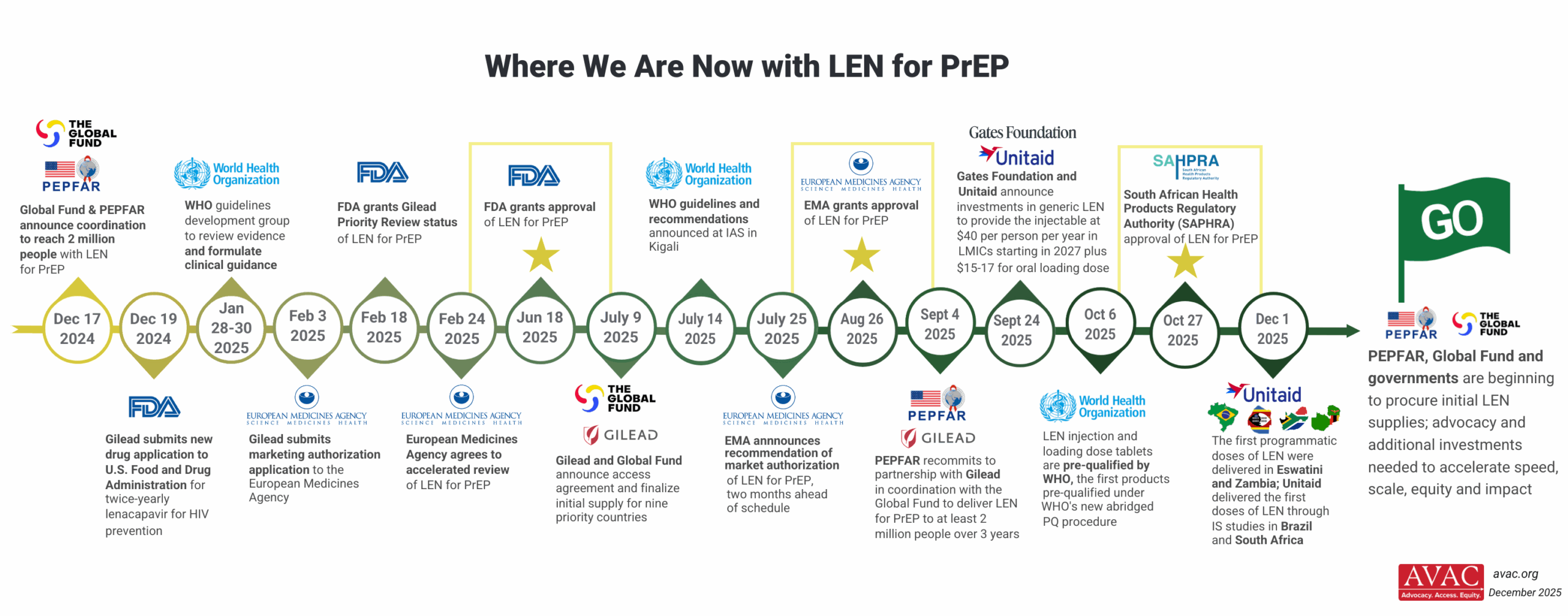
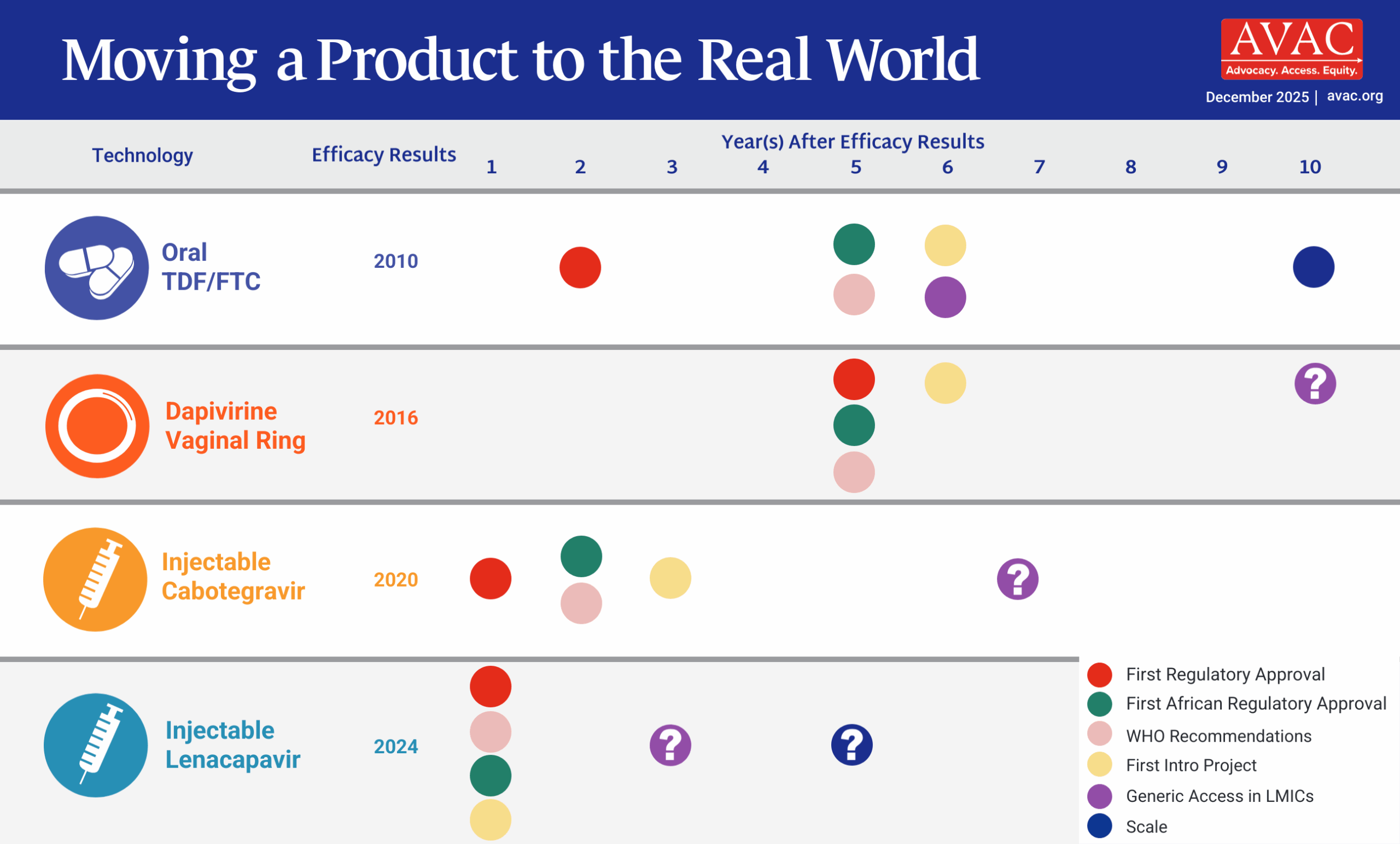
The Pathway to Access and Impact
This graphic outlines the critical steps for rolling out long-acting injectable PrEP products.
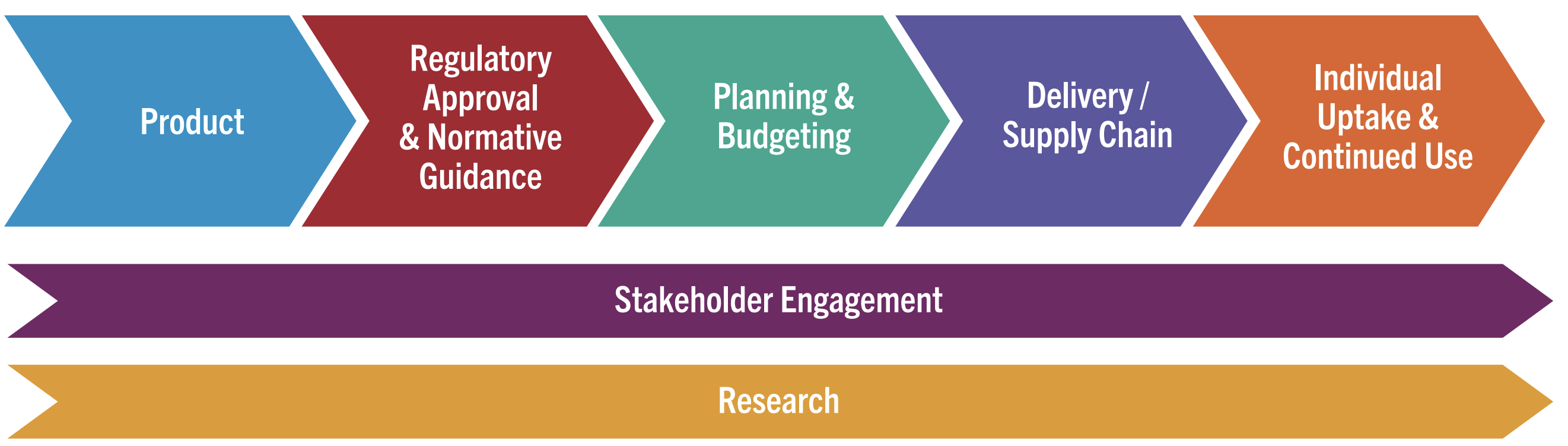
Learning Lessons, Accelerating Access, Reducing Time to Impact
Click for downloadable version or hover over the graphic above and click the three dots for the full screen version.