Coalition to Accelerate Access to Long-Acting PrEP

Purpose
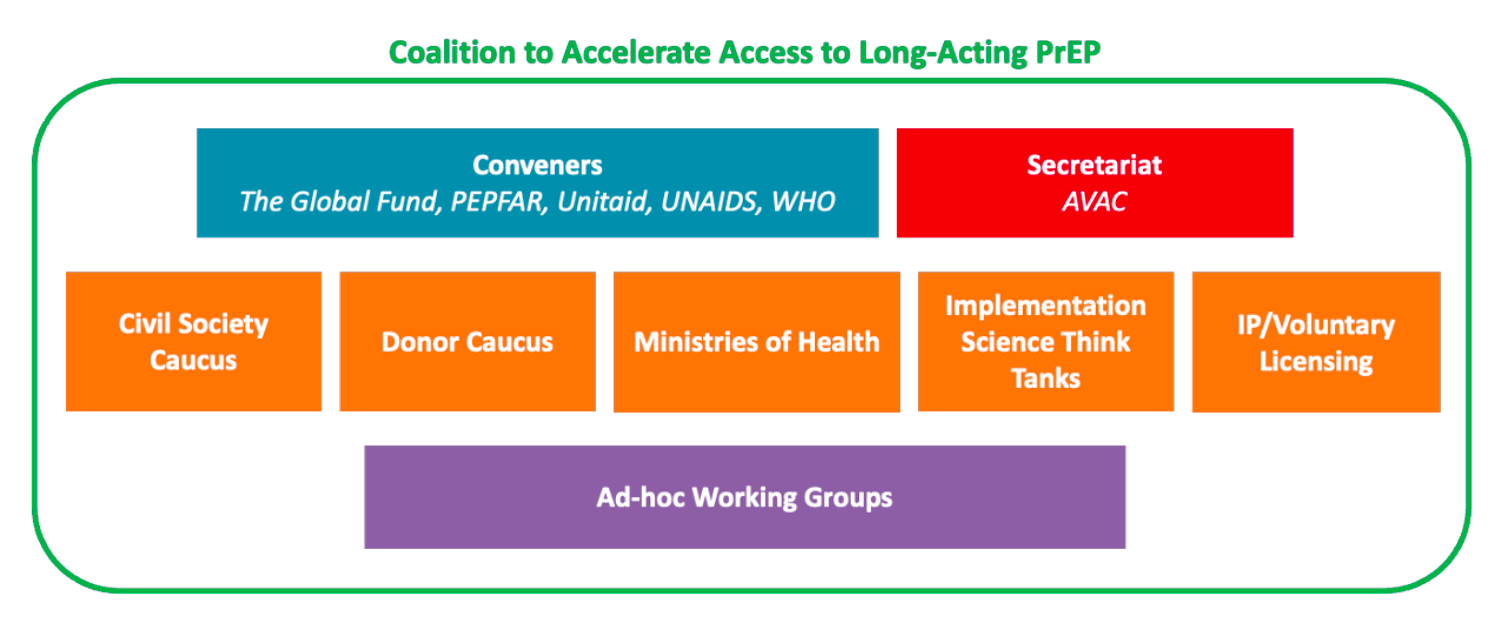
The Coalition to Accelerate Access to Long-Acting PrEP brings together donors, civil society, ministries of health, implementation science think tanks and partner organizations to ensure an accelerated, equitable, sustainable, and collaborative approach to optimizing access to new long-acting PrEP options. The Coalition is convened by the Global Fund, PEPFAR, Unitaid, UNAIDS and WHO, with AVAC as the Secretariat.
The Coalition’s objective is practical: to coordinate key stakeholder activities on long-acting PrEP access, including jointly developing strategies to identify and overcome access challenges for new PrEP options available now and those in the pipeline.
The Coalition is committed to transparency and will disseminate a quarterly update to chart progress overall and will periodically update the Plan for Accelerating Access and Introduction of Injectable CAB for PrEP and A Plan for Accelerating Access to Injectable LEN for PrEP with timelines and milestones across the full range of PrEP options. See also Gears of Lenacapavir for PrEP Rollout, outlining a focused plan for LEN for PrEP rollout over the next few years, specifying priorities by stakeholder and evaluating volume and pricing strategies.
For more information and/or with questions for the Coalition co-convenors, contact [email protected].
Long-Acting PrEP Status Update
The status update is revised on a quarterly basis by AVAC, Secretariat to the Coalition to Accelerate Access to Long-Acting PrEP, to synthesize the current status of long-acting PrEP products. This is done in service to the wider ecosystem.
Visit for graphics pertaining to the regulatory approvals, volumes, ongoing and planned implementation science studies, and non-profit prices for currently available long-acting PrEP products (currently injectable cabotegravir and dapivirine vaginal ring).
Coalition Report
- Coalition Fact Sheet
- Quarterly Coalition Report Q1 2024 (Full Report / Executive Summary Report)
Latest Updates
PrEP Power, a project led by Global Black Gay Men Connect (GBGMC), supported by the Global Key Population Advisory Group (KPAG), and with technical support from AVAC, is spearheading efforts to ensure KPs have equitable and effective access to the full range of HIV prevention options, including long-acting PrEP.
PrEP Power will coordinate market assessments, demand forecasting, human centered design research, community education, and demand generation to address barriers to long-acting PrEP access by KPs.
Gathering the right data and committing resources to fully understand how to deliver new prevention options, such as injectable cabotegravir (CAB), the dapivirine vaginal ring (DVR), and lenacapavir (LEN), and perhaps the monthly oral pill MK-8527 in the future if successful in the current trials, could increase effective HIV prevention coverage among KP communities.
Rationale
The Coalition to Accelerate Access to Long-Acting PrEP will urgently apply lessons learned from oral PrEP, create plans to overcome the unique challenges for new prevention options, with the aim that new, longer-acting PrEP options will be quickly and equitably accessible to all who need them, focused especially on low- and middle-income countries (LMICs).
More than 10 years ago, research showed oral PrEP was safe and effective in preventing HIV. In 2012, it was approved for use by the US Food and Drug Administration and subsequently recommended for all people at substantial risk of HIV by the World Health Organization (WHO). But the global health community moved too slowly and more than a decade later, only 5.67 million people have initiated oral PrEP use (cumulative number of initiations from 2012 to September 2023), a fraction of the estimated number of people who need it and could benefit from it. With new, longer-acting PrEP options reaching the market, the global health field needs to bring bold actions, global urgency, and coordinated partnerships to meet the challenges of product access, roll-out, and use. Product developers, policy makers, normative agencies, donors, program implementers, researchers, manufacturers, civil society, advocates, and communities all have critical roles to play. Strategic action is needed to ensure that science is translated into public health impact without unnecessary delay.