Long-Acting PrEP Status Update
Updated as of January 2025
This long-acting PrEP status update is revised on a quarterly basis by AVAC, Secretariat to the Coalition to Accelerate Access to Long-Acting PrEP, to synthesize the current status of long-acting PrEP products. This is done in service to the wider ecosystem. It is not a deliverable of the Coalition nor representative of the views of the individual institutions that make up the Coalition.
Below you will find graphics pertaining to the regulatory approvals, volumes, ongoing and planned implementation science studies, and non-profit prices for currently available long-acting PrEP products (currently injectable cabotegravir and dapivirine vaginal ring).
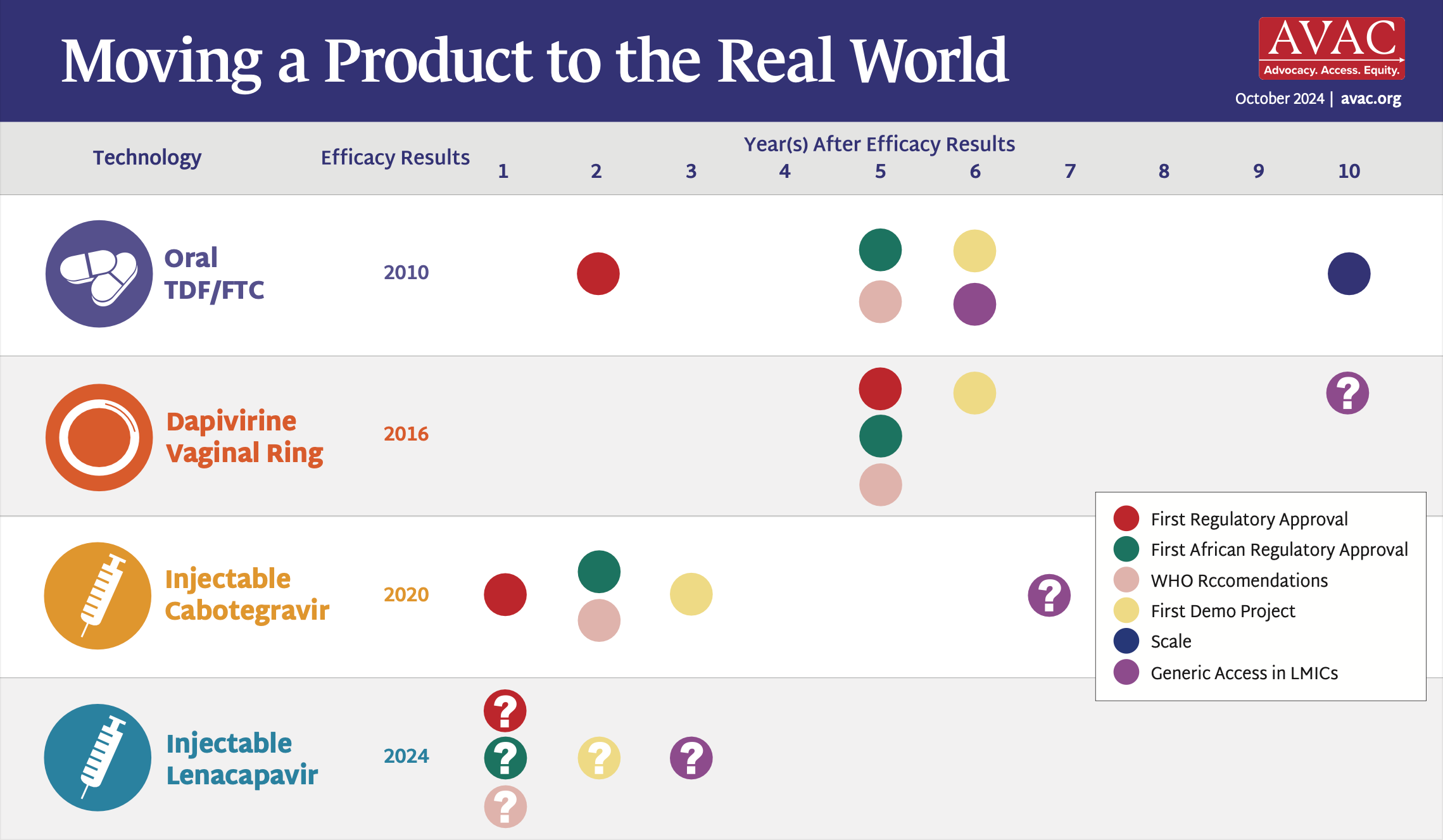
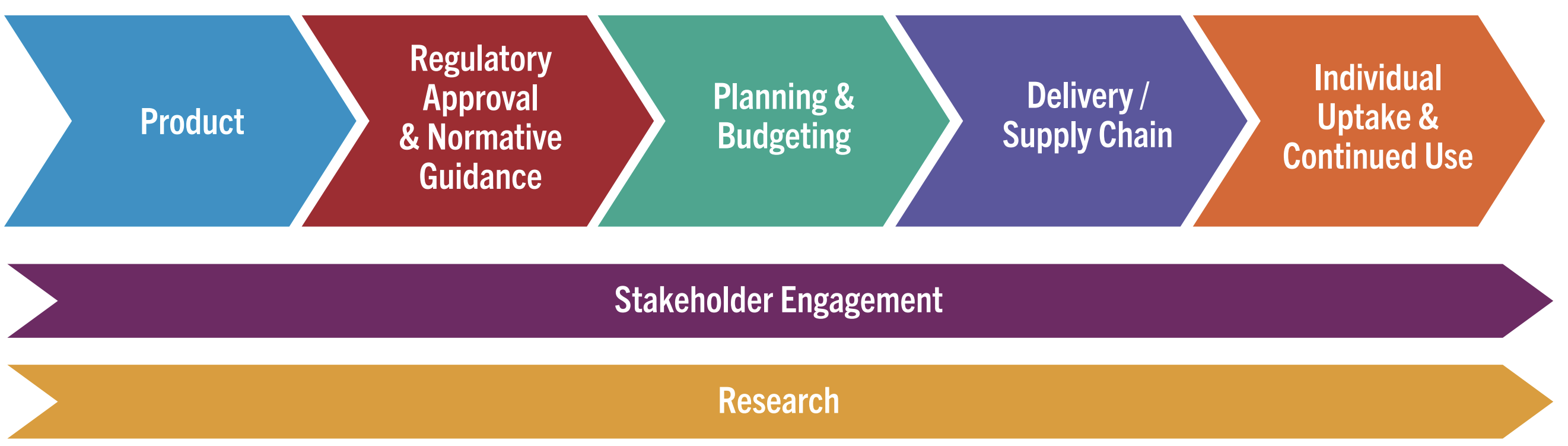
The Pathway to Access and Impact
This graphic outlines the critical steps for rolling out long-acting injectable PrEP products.
Snapshot: Current Status as of October 2024
| CAB | DVR | LEN | |
|---|---|---|---|
| Product (pricing, manufacturing, generics) | Current price form ViiV made public: £23.50/vial (approximately $180/year). Voluntary license granted from MPP to 3 generics—expected to market by 2027 | Current price from PopCouncil is approx. $156/year. MOU signed with Kiara Health to manufacture in South Africa but no clear timeline for local manufacturing | Gilead announced early review of PURPOSE 1 and 2 trial data in June and September. LEN was safe and effective in both trials, with no infections shown (PURPOSE 1) and a 96% lower HIV rate (PURPOSE 2). Gilead licensed six generic manufacturers to produce LEN for 120 countries |
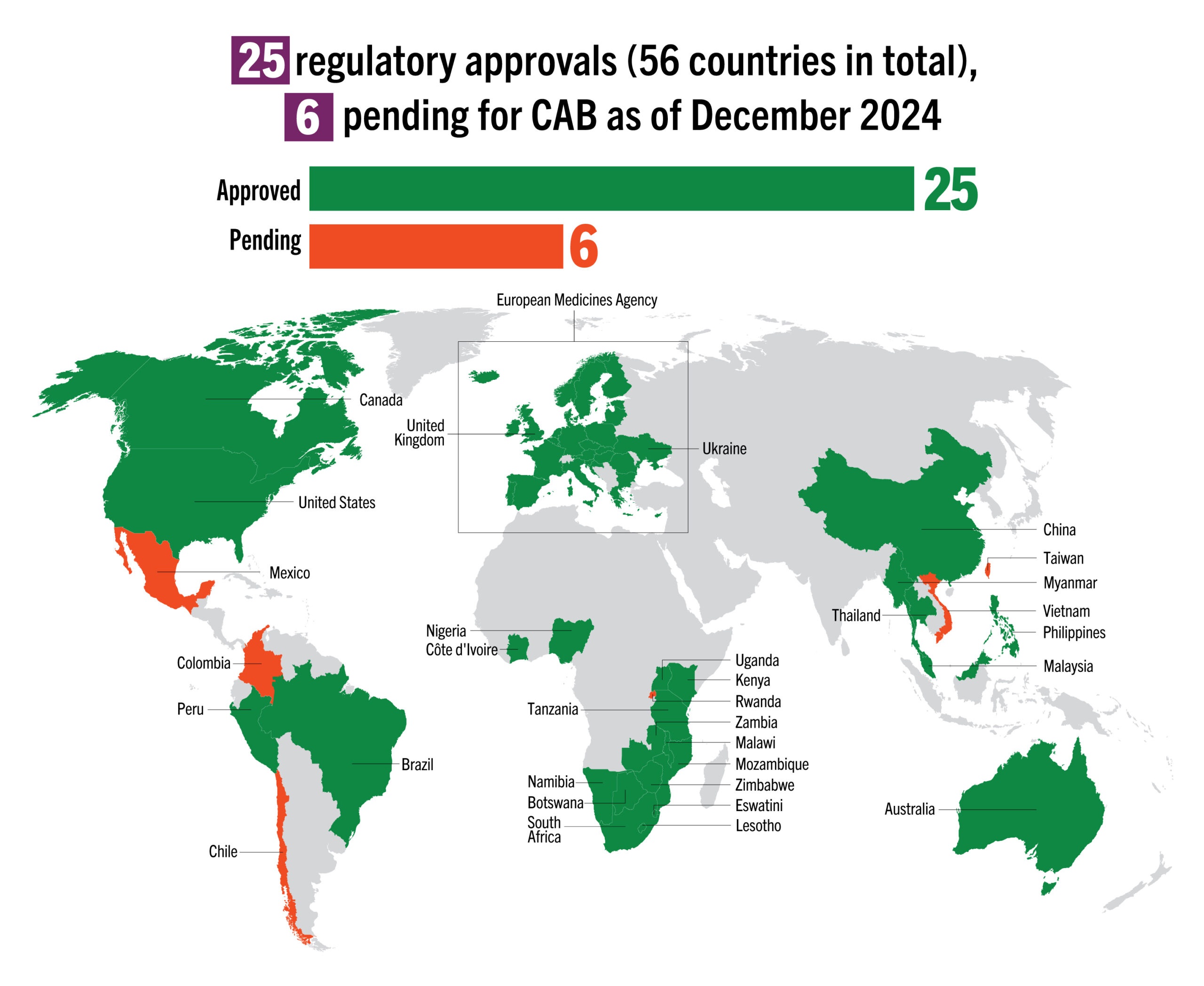
| Regulatory Approval & Normative Guidance | 25 regulatory approvals (including EMA); pending in 8 countries; WHO pre-qualification in 2023 | Approved in 11 countries; 2 pending review/appeal; WHO pre-qualification in 2021 | Possible regulatory submissions by early 2025, pending additional data and review |
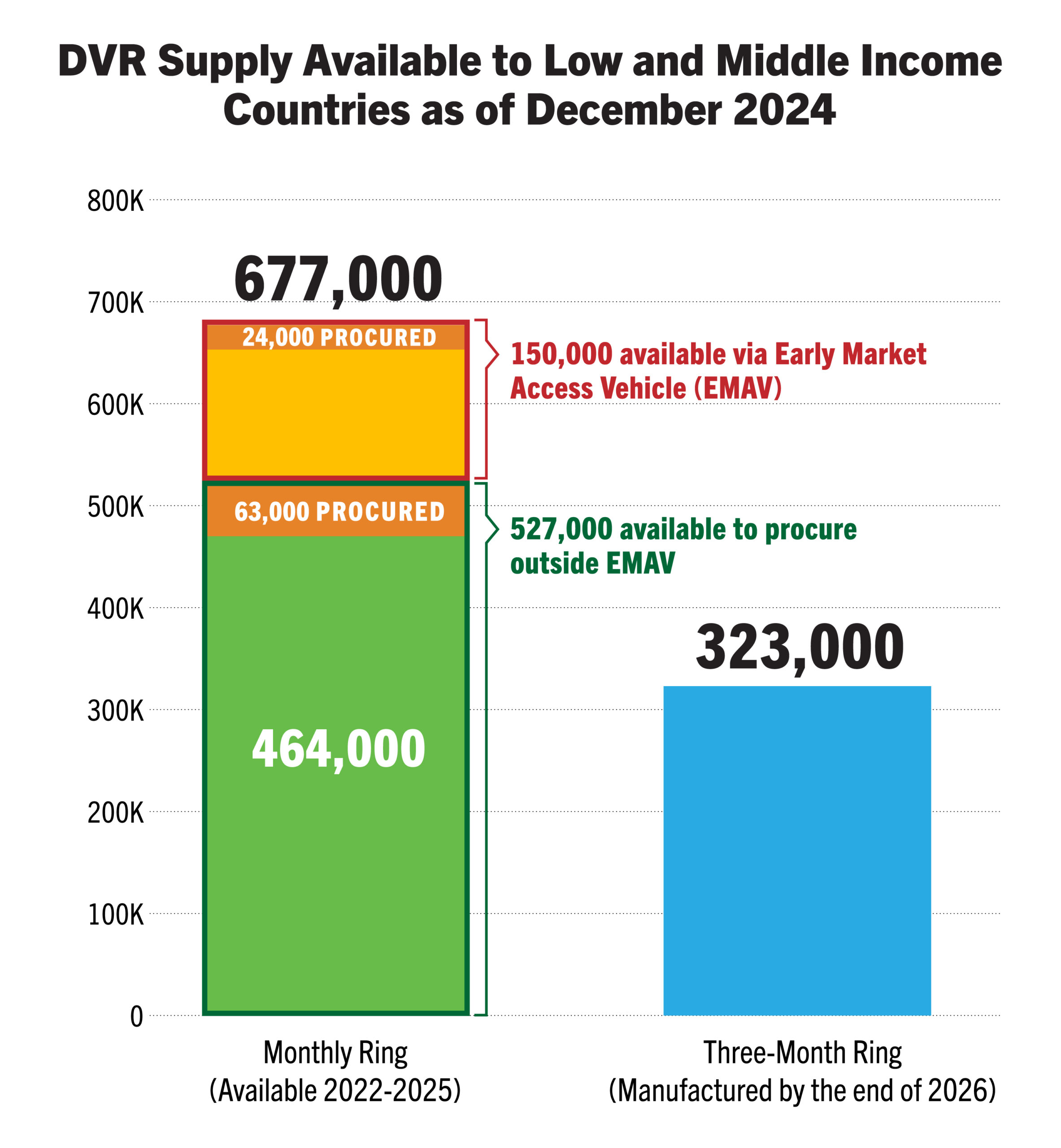
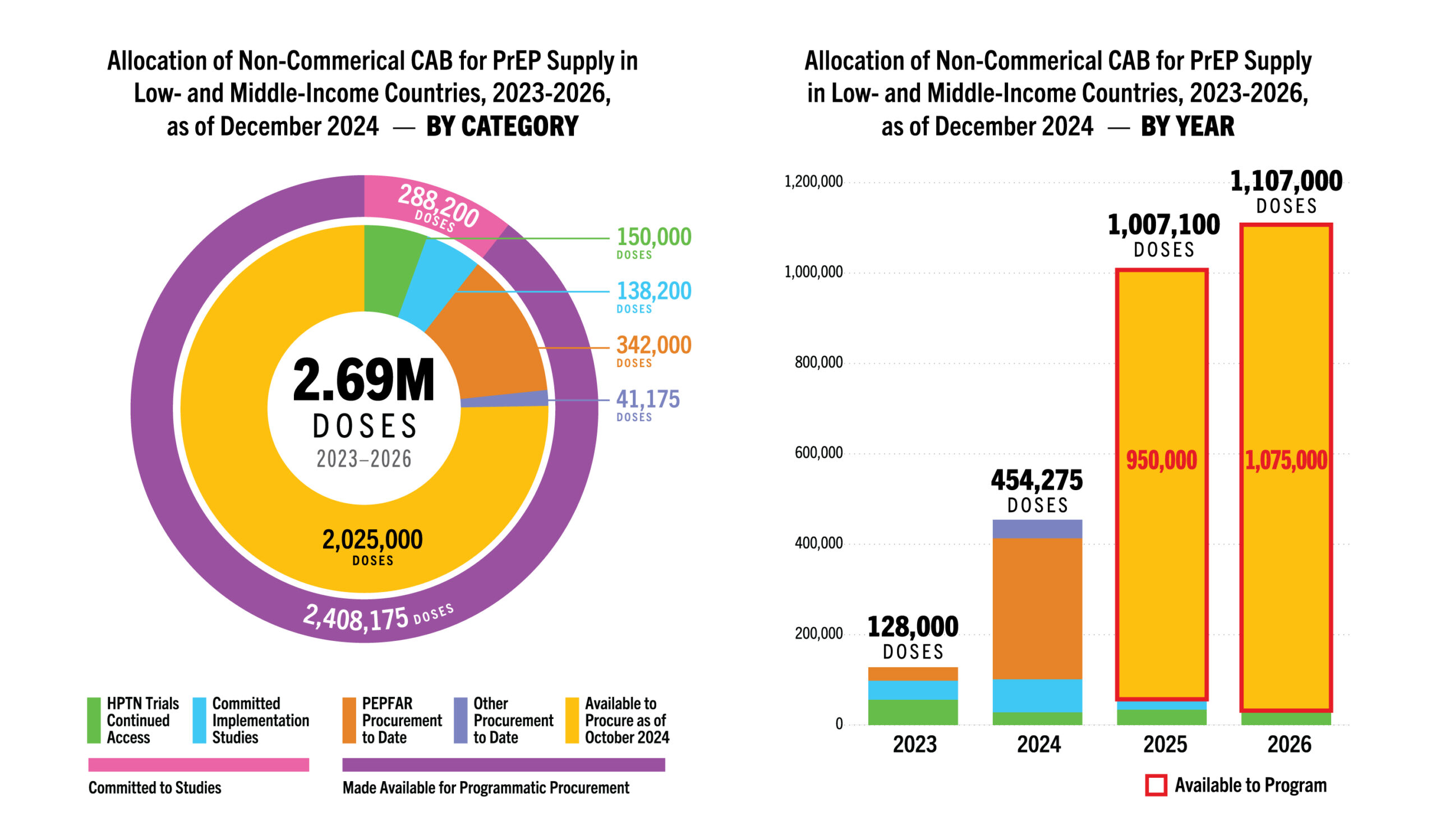
| Planning & Budgeting | Total 2023-2026 volume: updated to 2.64M doses available (study and programmatic supply) | 677k rings available 2022-2024, 323k to be manufactured by end of 2026. CIFF and Global Fund committed up to USD$2 million over 2024-2025, for purchase of approx. 150k DVRs | Gilead has stated capacity to manufacture up to ten million doses of lenacapavir by 2026—enough for 2.5 million potential lenacapavir users |
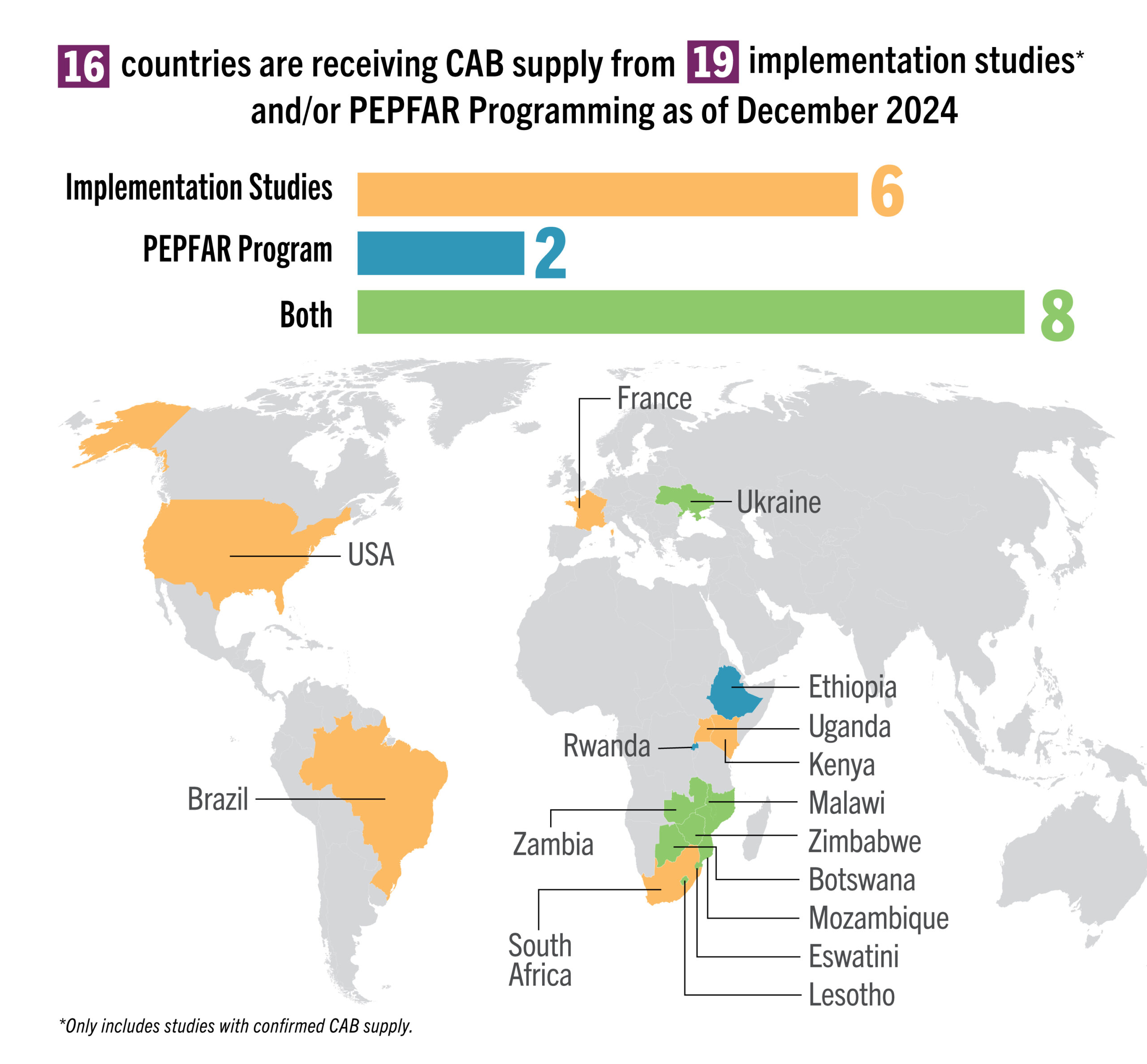
| Delivery & Supply Chain | Approx. 15,500 initiations as of Q3 2024 | Approx 1,860 initiations as of Q3 2024 | TBD during 2024/5, pending regulatory approvals and Gilead supplies |
| Stakeholder Engagement | Establishment of the Coalition’s Civil Society (CS) Caucus; facilitating reoccurring meetings with product developers; generic manufacturers; funders; and other stakeholders | ||
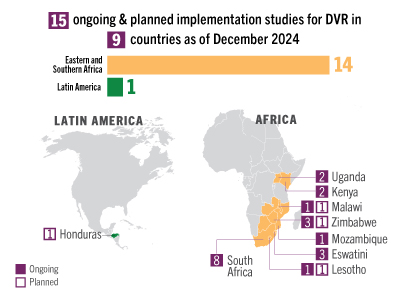
| Research | 39 implementation studies completed, ongoing or planned in 22 countries; findings from two US real-world evidence studies showed more than 99% effectiveness of CAB | 14 implementation studies completed, ongoing or planned in 9 countries. Positive data on 3-monthly ring presented at R4P | PURPOSE 3, 4 & 5 ongoing; develop additional implementation science agenda, as needed |
| Monitoring & Evaluation | Continued monitoring and assessment of initiations via trackers and think tanks. Understand country-specific product introduction issues to inform programmatic rollout | TBD during 2024/5 | |
Proposed Priorities for H2 2024 to H1 2025
| CAB | DVR | LEN | |
|---|---|---|---|
| Product (pricing, manufacturing, generics) | Collaborate with ViiV to understand procurement plans, build demand and accelerate generics progress | Collaborate with PopCouncil on price/volume for 2024/25 & plans for local mfg with Kiara; track development of 3-monthly & dual-purpose rings | Engage with Gilead now to encourage continued inclusivity and transparency on pricing and access. Work with six generic manufacturers with haste and in parallel to regulatory submissions. |
| Regulatory Approval & Normative Guidance | Monitor progress on the need to remove oral CAB development and registration from sublicence agreements | Advocate for additional submission in high-burden countries | Engage with Gilead to ensure timely sharing of PURPOSE 1 data and begin submission to WHO and regulatory agencies. WHO and national agencies to review data ASAP |
| Planning & Budgeting | Build demand in country and develop long-term demand forecast | Build demand in country and develop long-term demand forecast; understand what data PEPFAR may need to consider programmatic procurement | PEPFAR and the Global Fund to work with other donors and MoH to negotiate price and volume guarantees. MoHs to integrate into national guidelines ASAP |
| Delivery & Supply Chain | Track current implementation studies and share early insights; continue to identify and address evidence gaps | Track current implementation studies and share early insights; continue to identify and address evidence gaps | MoHs, policy makers and donors to collaboratively design a comprehensive introduction strategy to speed up introduction |
| Stakeholder Engagement | Create collective advocacy strategies, continue to integrate civil society perspectives and support implementation of HIV Prevention Choice Manifesto | Create collective commitment to expedite comprehensive access strategy by engaging in open conversations and engagement in Plan for LEN and upcoming documents | |
| Research | Ensure further studies are planned to research long-term effects, and continue to identify gaps in product introduction by country | Advocate for further research on long-term effects and use in conjunction with other prevention methods | Identify implementation science priorities that can be embedded in programmatic rollout and/or run in parallel in anticipation of approval & recommendations |
| Monitoring & Evaluation | Continue to coordinate modeling exercises; assess gaps in in product introduction by country. Push to advance a learning agenda for programmatic rollout | Continue to assess gaps in in product introduction by country. Push to advance a learning agenda for programmatic rollout | Anticipate gaps in in product introduction by country; push to advance a learning agenda for programmatic rollout |
Comparing Product Approvals, Volume, Implementation, and Price
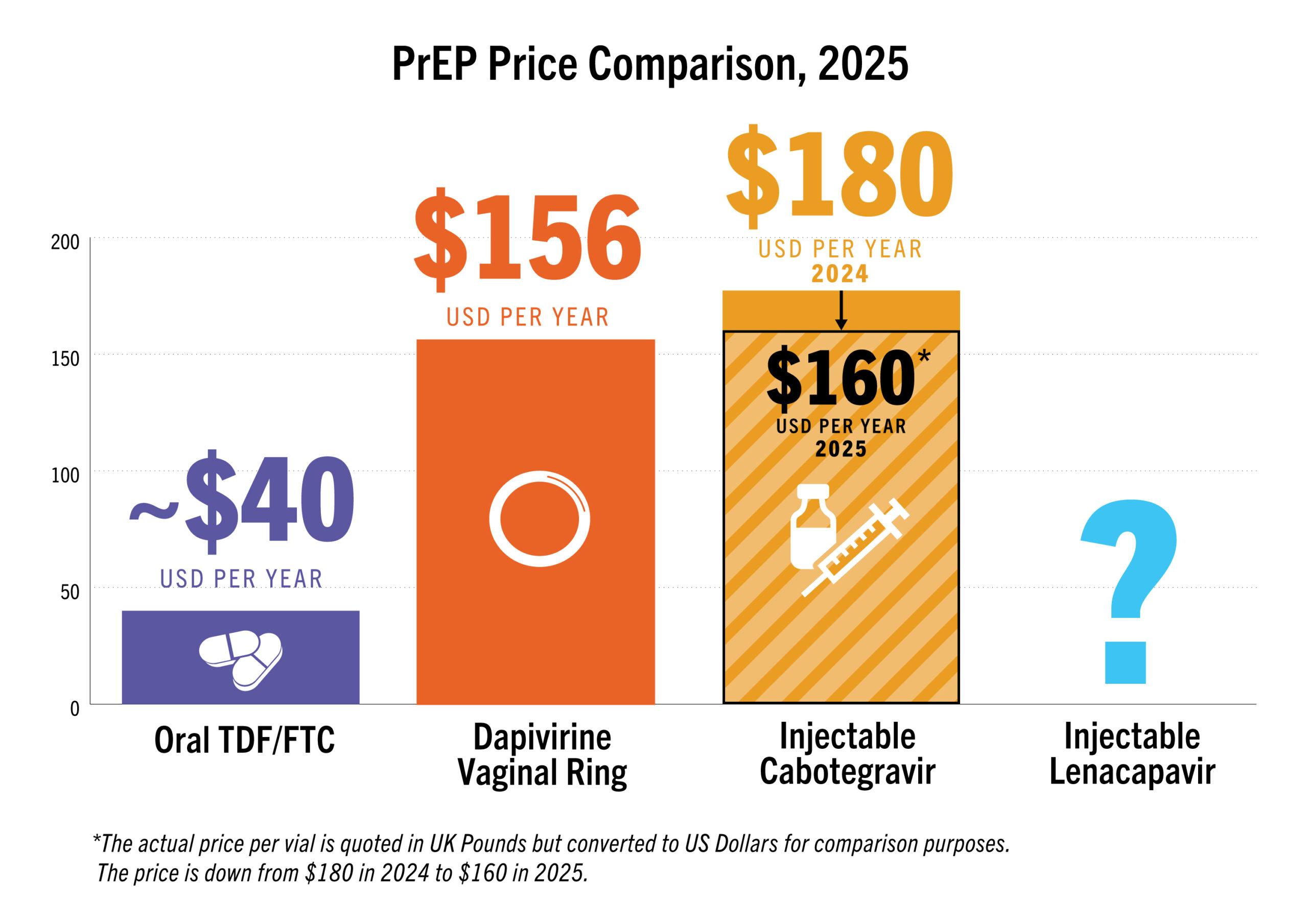
PrEP Price Comparison
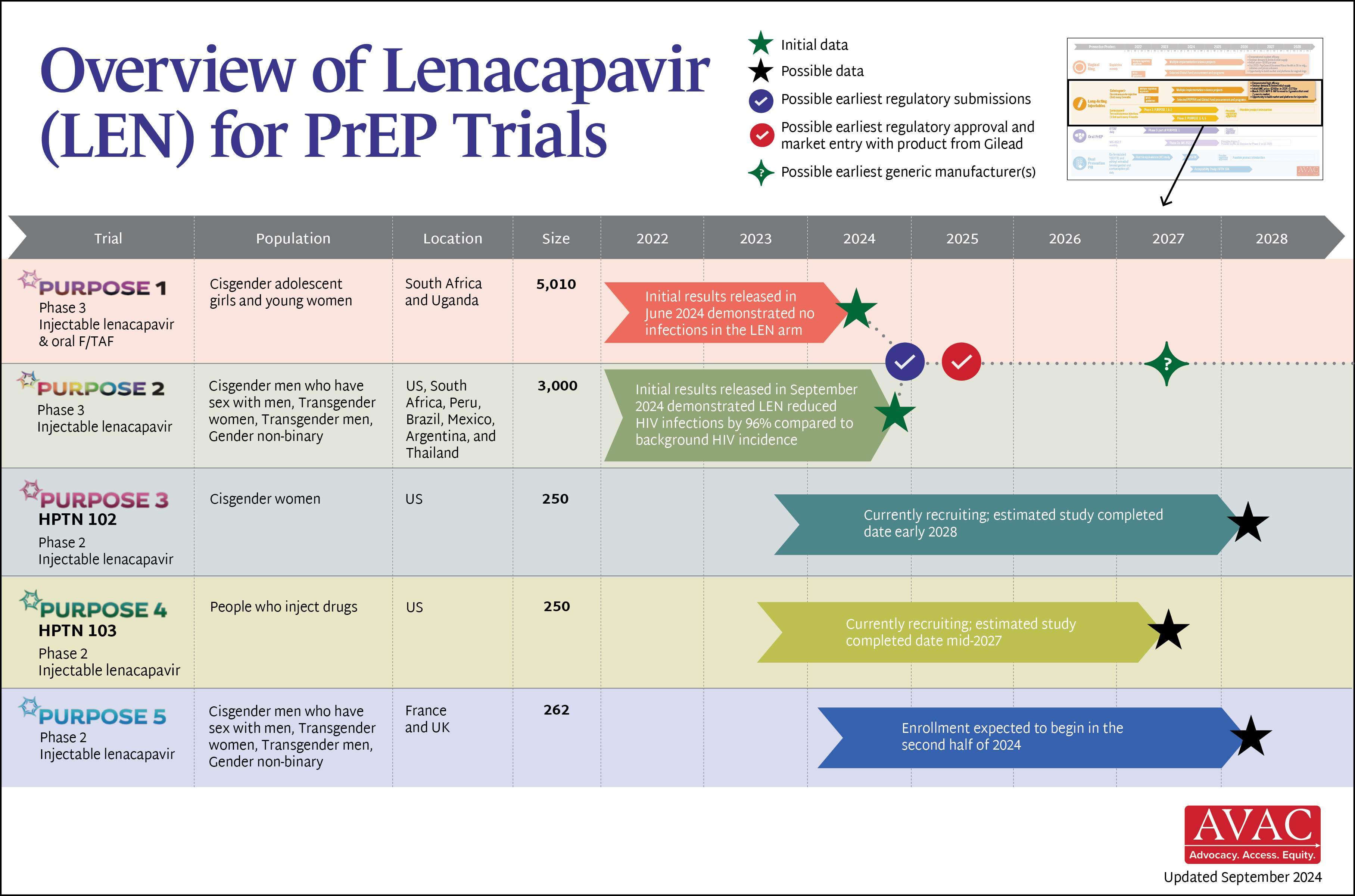
An Overview of Lenacapavir for PrEP Trials
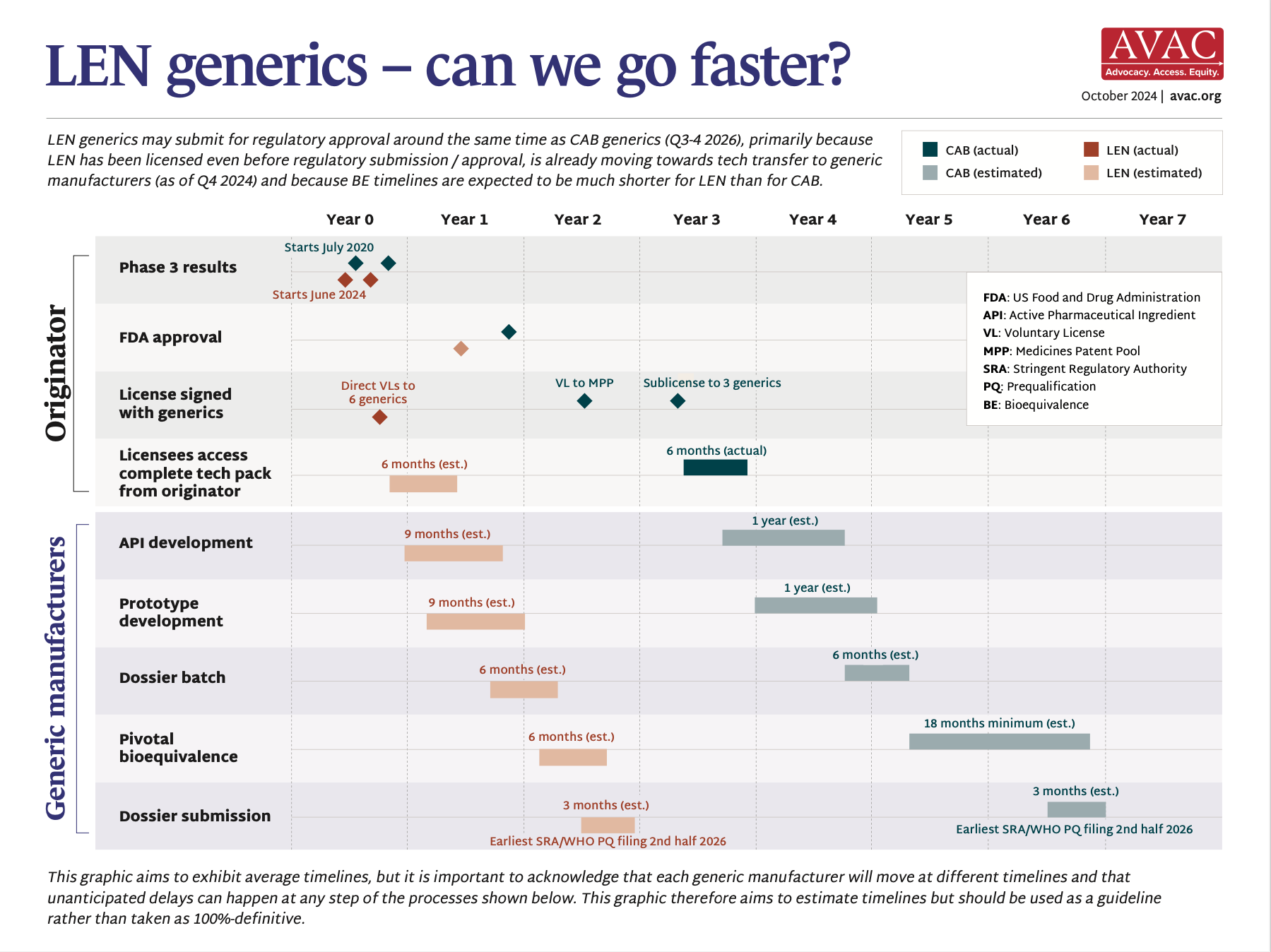
LEN Generics — Can we go faster?