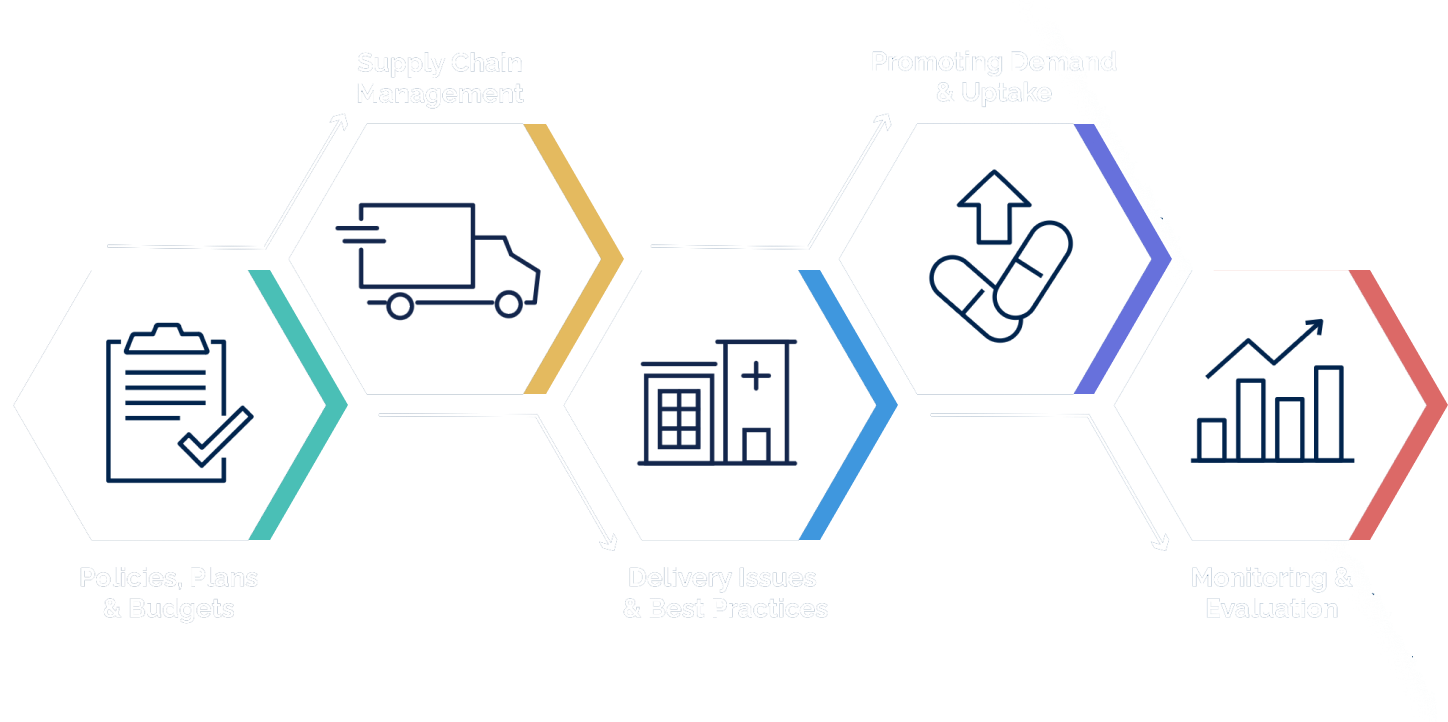
How to Deliver PrEP
Use our step-by-step framework for scaling up PrEP.
Learn MoreRecent data led by Dr. Jeanne Marrazzo show very high effectiveness of oral TDF/FTC for women with daily adherence and also for those who had consistently high adherence of at least four pills per week.

Promising growth in oral PrEP usage in Nigeria: Recent trends are very encouraging. Learn more or view global data.

Explore a curated collection of external resources and websites to further your knowledge and access to PrEP-related information. These links provide valuable insights, data, and more.
Accelerating introduction of new biomedical prevention products to help individuals, especially women, prevent HIV and other infectious diseases.
Supporting stakeholders looking to find community, increase their understanding on a topic and implement advocacy action around clinical research and implementation.
Working to understand potential product users, analyze market data, and convene decision-makers to more effectively design programs that increase access to prevention options.
The most up-to-date and comprehensive field-wide estimates for HIV prevention and R&D globally.
The first go-to resource for information on long-acting technologies patents and licenses.
It is a long established fact that a reader will be distracted by the readable content of a page when looking at its layout.